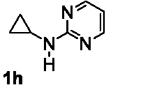
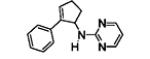
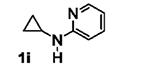
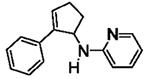
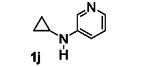
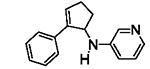
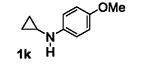
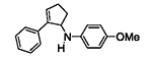
Table 5.
[3+2] Annulation of various aryl amines.[a]
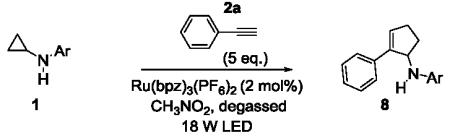
A solution of 1 (0.2 mmol), Ru(bpz)3(PF6)2 (2 mol%), and 2a (5 equiv.) in 2 mL CH3NO2 was degassed via freeze-pump-thaw cycles. The resulting solution was irradiated using an 18 W white LED.
Monitored by TLC.
Isolated yields.
Determined using GC-MS.