Abstract
Aim
To determine if children and adolescents who have obesity (Ob) or type 2 diabetes (T2DM) of relatively short duration have impaired cardiovascular function compared with lean subjects using 24-hour ambulatory blood pressure as a surrogate measure of evaluation.
Methods
We enrolled 100 African-Caribbean subjects (45 males/55 females), mean ages 14.4-15.2 years (range 11.8-18.5 years) and Tanner stage 4.2-4.8. Mean BMI for the Ob (n = 40), T2DM (n = 39) and lean (n = 21) groups were 40.3, 34.2 and 20.8, respectively (p < 0.01, Ob and T2DM vs. lean). Mean hemoglobin A1c in lean and Ob was 5.4 and 5.5% compared to 8.8% in T2DM (p < 0.001, T2DM vs. lean and Ob). Ambulatory blood pressure was recorded every 20 min over 24 h using Spacelabs 70207.
Results
Mean 24-hour, daytime and nighttime systolic blood pressure was significantly higher in Ob and T2DM compared with lean subjects (mean 24-hour 117 and 120 vs. 109 mm Hg; daytime 121 and 123 vs. 113 mm Hg; and nighttime 109 and 115 vs. 101 mm Hg; p < 0.01 for all time periods). The nocturnal systolic dip in Ob and T2DM did not differ from that of lean, whereas nocturnal diastolic dip decreased significantly in Ob and T2DM compared to lean (11.5 and 10.4 vs. 20.6 mm Hg; p < 0.01). Mean pulse pressure was significantly increased in the Ob and T2DM groups compared to lean subjects (51 and 54 vs. 45 mm Hg; p < 0.01).
Conclusion
Adolescent Ob and T2DM groups share adverse risk factors, which may be harbingers of adult cardiovascular events.
Key Words: Ambulatory blood pressure, Ambulatory blood pressure monitoring, Type 2 diabetes, Obesity, Children, Homeostasis model assessment of insulin resistance
Introduction
The obesity epidemic in the pediatric age group continues to be a global public health focus. The prevalence of obesity in the United States has increased 3-fold since 1963 and especially affects non-Hispanic Black and Hispanic children and adolescents [1]. Longitudinal studies have firmly established the relationship of childhood obesity with components of metabolic syndrome, such as insulin resistance (IR), dyslipidemia and hypertension. Since childhood obesity tends to track into adulthood, obese children and adolescents appear to be at a higher risk of developing type 2 diabetes mellitus (T2DM) and hypertension as they age, thereby increasing their risk of developing cardiovascular disease (CVD) [2,3].
As high blood pressure (BP) is one of the most important modifiable risk factors for CVD, the increasing prevalence of hypertension in children is of concern. Postmortem studies have demonstrated increased atherosclerosis in adolescents with higher BP levels [4]. Therefore, early and accurate detection of hypertension in childhood is important in preventing CVD. Ambulatory BP monitoring (ABPM) has been shown to be superior to casual BP for the detection of subjects at high risk for CVD [5,6]. Not only does ABPM reduce observer errors, it also provides information on dipping status (nocturnal fall in BP), morning BP surges, white coat hypertension, masked hypertension (office BP <140/90 mm Hg, while ABPM or home BP readings are in the hypertensive range) and BP load. Moreover, in children and adolescents, masked hypertension may be a precursor of sustained hypertension and left ventricular hypertrophy [7]. Blunted nocturnal dipping is now a well-established prognostic risk factor for unfavorable cardiovascular outcomes in adults [8], but its prognostic value has not been well established in the pediatric population. Many obese children have only isolated nocturnal hypertension [9], which further emphasizes the need for ABPM to better predict CVD outcome.
Because obese and diabetic children appear to be at a higher risk for the development of CVD, an early and accurate assessment of BP is of utmost importance. The purpose of our study was to perform ABPM in T2DM and nondiabetic, obese children and adolescents, who are presumed to be at higher risk of CVD, compared with lean controls.
Methods
A total of 100 African-Caribbean children, aged 14-16 years, were enrolled, including 21 lean, 40 obese and 39 children with T2DM. The inclusion criterion was a body mass index (BMI) between the 25th and 75th percentile in the normal weight group and a BMI >95th percentile in the obese group, based on 2000 Centers for Disease Control growth charts. Children in the T2DM group were diagnosed according to American Diabetes Association guidelines. Children in the lean and obese groups were free of diabetes, cardiovascular and other chronic diseases. No T2DM children were on insulin therapy, and no participant was receiving lipid-lowering agents. Informed written consent was obtained in accordance with the guidelines of Kings County Medical Center and State University of New York, Downstate Medical Center Institutional Review Boards for human subjects.
Anthropometric Measurements
A pediatrician completed a detailed history and physical examination on each participant. Pubertal status based on Tanner staging was determined by assessing breast development in girls and testicular volume in boys. Height and weight were measured and expressed as percentiles using Centers for Disease Control norms for the child's age and sex. BMI was calculated as weight (kg) divided by height squared (m2).
Laboratory Measurements
Venous blood samples were obtained in the morning after an overnight fast in all patients for measurements of serum glucose, insulin and lipid profile. Glucose and insulin values were used to calculate fasting IR according to the homeostasis model assessment of insulin resistance (HOMA-IR), with HOMA-IR calculated as fasting insulin (μU/ml) × fasting glucose (mmol/l)/22.5. Based on receiver operating characteristic analysis, a HOMA value >3.16 was considered diagnostic of IR in children and adolescents [10].
Ambulatory Blood Pressure Monitoring
ABPM was performed with a Spacelabs 70207 monitor (Spacelabs Medical, Redmond, Wash., USA). The measurements were obtained with a BP cuff on the nondominant arm every 20 min over a 24-hour period. All ABPM recordings were performed on school days. Daytime and nighttime were determined for each subject from diaries of sleep and wake times [11]. The upper limits for daytime systolic (SBP) and diastolic BP (DBP) were indexed to the 95th percentile for age, gender and height. ABPM recordings were required to have a minimum of 40 readings with ≤2 h between successful readings. Hypertension was defined as SBP and DBP >95th percentile for gender, age and height. BP load was calculated as the percentage of valid ambulatory BP measurements for a given period above the set 95th percentile of BP for age, gender and height [12]. A normal nocturnal fall (dip) in BP was defined as ≥10% difference between daytime and nighttime BP values. The percentage of nocturnal BP dipping was calculated as [(daytime BP – nighttime BP)/daytime BP] × 100.
Statistical Analysis
All statistical operations were performed using JMP software (SAS Institute, Cary, N.C., USA). All values were expressed as mean ± standard error of the mean. Differences in group means were determined by analysis of covariance (ANCOVA). Univariate associations between variables were analyzed by calculating the Pearson correlation coefficient (r). Statistical significance was set at a p value of <0.05.
Results
Demographics (table 1)
Table 1.
Baseline characteristics
| Parameter | Lean | Obese | T2DM |
|---|---|---|---|
| Number | 21 | 40 | 39 |
| Gender (male/female) | 9/12 | 20/20 | 15/24 |
| Age, years | 14.9±0.4 | 14.6±0.3 | 15±0.4 |
| Tanner stage | 4.3±0.1 | 4.6±0.1 | 4.6±0.2 |
| DM duration, months | – | – | 23±5 |
| Antihypertensive treatment, n | 0 | 1 | 17 |
| Height, in | 63±1 | 65±1 | 66±1* |
| Weight, lb | 120±7 | 243±9** | 214±9** |
| BMI | 20.8±0.6 | 40.3±1** | 34.2±1.1** |
| Hemoglobin A1c, % | 5.4±0.2 | 5.5±0.2 | 8.8±1**, † |
p < 0.05 versus lean
p < 0.01 versus lean
p < 0.01 versus obese.
All the participants were of African-Caribbean descent. Mean ages and Tanner stages were similar in each group, ranging from 14.4 to 15.2 years and from stage 4.2 to 4.8, respectively. The T2DM group had more females than males. The mean duration of diabetes in the T2DM group was less than 2 years (23 ± 5 months). Most of the T2DM subjects were on oral hypoglycemic agents, mainly metformin. Of 39 T2DM subjects, 17 were receiving antihypertensive agents, mostly ACE inhibitors.
As expected, obese and T2DM subjects were significantly heavier than lean subjects, and their mean BMI greatly exceeded the 95th percentile for age and gender compared to lean subjects (40.3 ± 1 and 34.2 ± 1 vs. 20.8 ± 1; p < 0.01). Mean glycosylated hemoglobin levels were 8.8 ± 1% in T2DM compared with 5.5 ± 0.1 and 5.4 ± 0.2% (p < 0.01) in obese and lean subjects, respectively.
Insulin Resistance and Glycemic Control
Fasting serum glucose was significantly higher in T2DM subjects (147 ± 22 mg/dl) compared to lean and obese subjects (83 ± 3 and 87 ± 2 mg/dl; p < 0.01). Fasting plasma insulin levels were 32 ± 6 and 31 ± 5 μU/ml in the obese and T2DM groups (reference range: 0-23 μU/ml). Plasma insulin levels were not obtained in lean subjects as they were not expected to have insulin resistance. HOMA-IR values in obese and T2DM groups were 5.9 ± 1.5 and 11.6 ± 7.6, respectively (table 2).
Table 2.
Serum biochemical measures
| Parameter | Lean | Obese | T2DM |
|---|---|---|---|
| Cholesterol, mg/dl | 158±9 | 168±5 | 175±8 |
| Triglycerides, mg/dl | 109±19 | 104±7 | 118±9 |
| HDL cholesterol, mg/dl | 52±6 | 44±2 | 44±1 |
| LDL cholesterol, mg/dl | 89±8 | 107±5** | 105±7** |
| Glucose, mg/dl | 83±3 | 87±2 | 147±22**, † |
| Insulin levels, μU/ml | – | 32±6 | 31±5 |
| HOMA-IR | – | 5.9±1.5 | 11.6±7.6 |
p < 0.01 versus lean
p < 0.01 versus obese.
Serum Lipids
Total cholesterol, triglyceride and HDL cholesterol levels did not differ between groups. LDL cholesterol was significantly higher in obese and T2DM subjects (table 2).
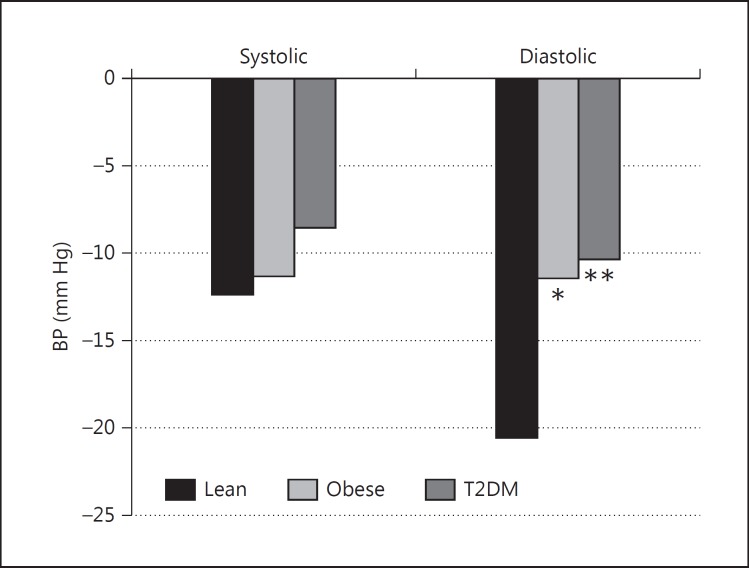
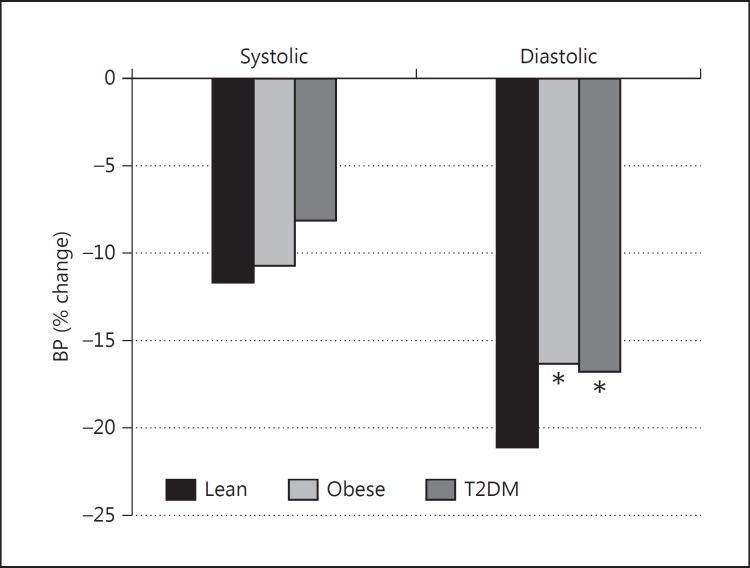
Nocturnal Dip (fig. 1, 2)
Fig. 1.
Nocturnal fall in BP. * p < 0.05 versus lean; ** p < 0.01 versus lean.
Fig. 2.
Percentage nocturnal fall in BP. * p < 0.05 versus lean.
Mean nocturnal SBP fell by 12.4, 11.4 and 8.6 mm Hg, in lean, obese and T2DM subjects, respectively, but between-group differences were not significant. Mean nocturnal DBP fell by 20.6, 11.5 and 10.4 mm Hg for lean, obese and T2DM subjects, respectively. The differences between lean, obese and T2DM subjects were significant (obese vs. lean: p < 0.05; DM vs. lean: p < 0.01). Although the percentage fall in nocturnal SBP was greater in the lean group, the between-group difference did not reach significance (11.8% in lean and 10.8 and 8.2% in obese and T2DM subjects, respectively). However, the percentage nocturnal DBP fall was significantly greater in lean subjects than in the obese and T2DM groups (21.2% for lean vs. 16.4 and 16.9% for obese and T2DM subjects, respectively; p < 0.05).
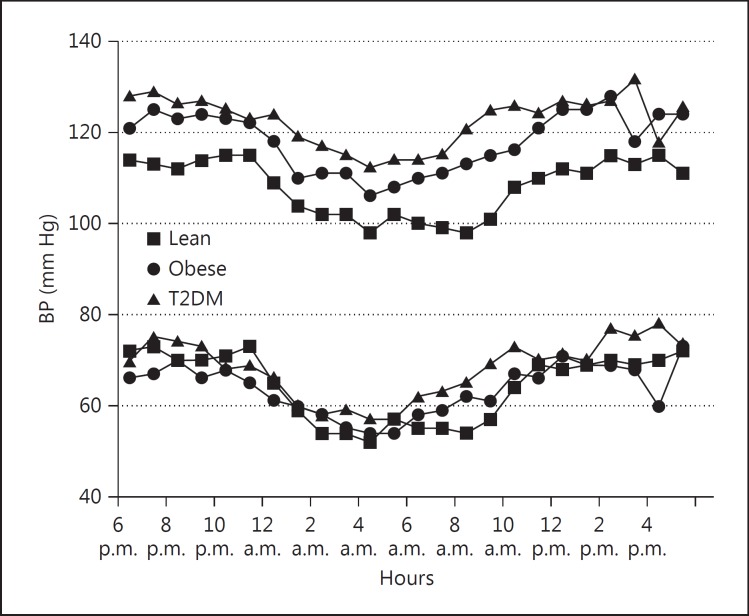
Twenty-Four-Hour ABPM (fig. 3)
Fig. 3.
Twenty-four-hour ambulatory BP.
Mean 24-hour, daytime and nocturnal SBP was significantly higher in the obese and T2DM groups than in the lean group (mean 24-hour SBP: 117 ± 2 and 120 ± 1 vs. 109 ± 2 mm Hg; daytime: 121 ± 2 and 123 ± 1 vs. 113 ± 2 mm Hg; nighttime: 109 ± 2 and 115 ± 2 vs. 101 ± 2 mm Hg; p < 0.01 for all time periods). Although DBP was unchanged, mean daytime and nighttime pulse pressures were significantly increased in the obese and T2DM groups (51 and 54 vs. 45 mm Hg; p < 0.01). Mean 24-hour SBP correlated with BMI for all subjects (r = 0.352; p < 0.001). Both the percentage of SBP readings exceeding the 95th percentile for age and height and the percentage of time the readings were elevated, were significantly greater in obese and T2DM subjects at all time periods (tables 3, 4).
Table 3.
ABPM data
| Parameter | Lean | Obese | T2DM |
|---|---|---|---|
| SBP, mm Hg | |||
| Mean (24 h) | 109±2 | 117±2** | 120±1** |
| Day | 113±2 | 121±2** | 123±1** |
| Night | 101±2 | 109±2* | 115±2** |
| DBP, mm Hg | |||
| Mean (24 h) | 64±1 | 64±1 | 67±1 |
| Day | 70±1 | 69±1 | 70±1 |
| Night | 55±1 | 58±1 | 61±1 |
| Heart rate, bpm | |||
| Mean (24 h) | 82±3 | 85±2 | 85±2 |
| Day | 87±4 | 88±2 | 89±2 |
| Night | 73±3 | 78±2 | 77±1 |
p < 0.05 versus lean
p < 0.01 versus lean.
Table 4.
Percent of time BP >95th percentile for age and height
| Parameter | Lean | Obese | T2DM |
|---|---|---|---|
| SBP, mm Hg | |||
| Mean | 6±2 | 20±5** | 25±4** |
| Day | 6±2 | 17±5** | 20±4** |
| Night | 6±3 | 24±5** | 32±5** |
| DBP, mm Hg | |||
| Mean | 5±1 | 6±1 | 8±2 |
| Day | 6±2 | 7±2 | 8±2 |
| Night | 2±1 | 3±1 | 6±2 |
p < 0.01 versus lean.
Discussion
Our findings indicate that daytime, nocturnal and 24-hour mean SBP are significantly higher in nondiabetic obese and diabetic children and adolescents as compared to lean subjects matched for age and sexual maturation; for all subjects, the mean SBP correlated with BMI. These findings and the association of SBP with BMI are in strong agreement with cross-sectional studies conducted worldwide showing that both office and ambulatory SBP are positively related to BMI in obese adolescents and children [13,14,15]. Although higher SBPs and DBPs were noted at all time periods in diabetic and obese children, between-group differences did not reach statistical significance. Both the proportion of 24-hour mean day and night BP readings and the percentage of time exceeding the 95th percentile for age and height were greater in diabetic and obese subjects compared to lean subjects, suggesting that both obese youngsters without diabetes and diabetic youngsters carry a substantially greater left ventricular load early in life and therefore are at a higher risk of cardiovascular complications than their nonobese, nondiabetic counterparts. In diabetic subjects receiving ACE inhibitor-based drug therapy, the achieved BP was similar to that of diabetic youngsters not receiving antihypertensive medication.
In our study, the nocturnal fall in DBP was attenuated in obese and diabetic subjects, compared to the normal dipping pattern in lean subjects. Although the nocturnal DBP dip was blunted more in the diabetic compared to the obese group, the difference was not significant. In contrast to these findings, diabetic and obese children and adolescents, mostly girls, showed smaller nocturnal falls in SBP compared to the lean subjects [16]. Among adults, nondipping has been seen in essential hypertension, overweight or obese persons, older-age individuals and in those with a family history of premature CVD. Persistent nondippers have been reported to be at a greater risk of developing increases in left ventricular mass index, interventricular septum thickness and left atrium and aortic root diameters compared with subjects with a normal dipping pattern, despite similar mean 24-hour values [17]. In a recent meta-analysis, nocturnal BP has been shown to be superior to daytime BP in predicting both cardiovascular events and total mortality [18]. Our findings of higher daytime and nocturnal SBP load and blunted nocturnal diastolic dip in obese and diabetic children may be linked to high insulin levels and HOMA index, surrogate markers of IR. IR has been demonstrated to be associated with overactivity of the central sympathetic nervous system, contributing to increased peripheral resistance, reduced baroreceptor function and activation of renal tubular sodium reabsorption in the kidney. The renal effects involve activation of the renin-angiotensin-aldosterone system, resulting in volume overload and higher BP [19,20,21].
Furthermore, in the vascular endothelium, IR is associated with a reduced local vasodilator response to insulin [22,23]. Acute administration of insulin has been shown to have vasodilatory effects. In normal men, infusion of physiologic doses of insulin decreases the augmentation index within 1 h, consistent with increased distensibility or vasodilatation of large arteries, whereas the vasodilatory response to insulin in patients with T2DM occurred after 2 h, suggesting that the delayed insulin action due to IR could predispose patients with diabetes to develop systolic hypertension [24].
In addition, puberty is also known to be associated with decreased insulin sensitivity [25]. There was a trend towards slightly more advanced Tanner stages in our obese and T2DM subjects as compared to lean subjects, although the differences were not significant.
Our study is limited by its cross-sectional study design, which does not allow an appraisal of the long-term cardiovascular outcomes of elevated SBP in children and adolescents. In addition, our study population was confined to obese and diabetic Caribbean youth, which may limit the extrapolation of results to the general population. However, we would not expect Caribbeans to differ substantially from other ethnic groups. It is possible that other factors, not accounted for in this study, such as salt intake and obstructive sleep apnea might have contributed to the BP levels and impaired nocturnal dipping in diabetic and obese subjects.
Conclusions
Young persons with early T2DM and nondiabetic obesity have increased 24-hour mean SBP, absence of normal nocturnal diastolic dip and widened pulse pressure compared with lean controls matched for age and sexual maturation. The strong correlation of body size and BP in obese and T2DM youth may reflect higher IR in these groups. The adolescent T2DM and obese groups share adverse cardiovascular risk factors, which may be harbingers of adult cardiovascular events.
Disclosure Statement
The authors declare no conflicts of interest and have no financial disclosures.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 3.Raitakari OT, Juonala M, Viikari JS. Obesity in childhood and vascular changes in adulthood: insights into the Cardiovascular Risk in Young Finns Study. Int J Obes. 2005;29(suppl 2):S101–S104. doi: 10.1038/sj.ijo.0803085. [DOI] [PubMed] [Google Scholar]
- 4.Tracy RE, Newman WP, 3rd, Wattigney WA, Srinivasan SR, Strong JP, Berenson GS. Histologic features of atherosclerosis and hypertension from autopsies of young individuals in a defined geographic population: the Bogalusa Heart Study. Atherosclerosis. 1995;116:163–179. doi: 10.1016/0021-9150(95)05525-2. [DOI] [PubMed] [Google Scholar]
- 5.Flynn JT. Ambulatory blood pressure monitoring in children: imperfect yet essential. Pediatr Nephrol. 2011;26:2089–2094. doi: 10.1007/s00467-011-1984-9. [DOI] [PubMed] [Google Scholar]
- 6.Urbina E, Alpert B, Flynn J, Hayman L, Harshfield GA, Jacobson M, Mahoney L, McCrindle B, Mietus-Snyder M, Steinberger J, Daniels S, American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee Ambulatory blood pressure monitoring in children and adolescents: recommendations for standard assessment. Hypertension. 2008;52:433–451. doi: 10.1161/HYPERTENSIONAHA.108.190329. [DOI] [PubMed] [Google Scholar]
- 7.Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, Redon J, Staessen JA. Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension. 2005;45:493–498. doi: 10.1161/01.HYP.0000160320.39303.ab. [DOI] [PubMed] [Google Scholar]
- 8.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 9.Westerstahl M, Marcus C. Association between nocturnal blood pressure dipping and insulin metabolism in obese adolescents. Int J Obes (Lond) 2010;34:472–477. doi: 10.1038/ijo.2009.181. [DOI] [PubMed] [Google Scholar]
- 10.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:500–503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 11.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 12.Soergel M, Kirscstein M, Busch C, Danne T, Gellerman J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1,141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 13.Lurbe E, Invitti C, Torro I, Maronati A, Aguilar F, Sartorio G, Redon J, Parati G. The impact of the degree of obesity on the discrepancies between office and ambulatory blood pressure values in youth. J Hypertens. 2006;24:1557–1564. doi: 10.1097/01.hjh.0000239291.32883.e3. [DOI] [PubMed] [Google Scholar]
- 14.Schiel R Beltschikow, Kramer G, Stein G. Overweight, obesity and elevated blood pressure in children and adolescents. Eur J Med Res. 2006;11:97–101. [PubMed] [Google Scholar]
- 15.Shi Y, de Groh M, Morrison H. Increasing blood pressure and its associated factors in Canadian children and adolescents from Canadian Health Measures Survey. BMC Public Health. 2012;12:388. doi: 10.1186/1471-2458-12-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Framme J, Dandardt F, Marild S, Osika W, Wahrborg P, Friberg P. 24-h systolic blood pressure and heart rate recordings in lean and obese adolescents. Clin Physiol Funct Imaging. 2006;26:235–239. doi: 10.1111/j.1475-097X.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 17.Cuspidi C, Meani S, Salerno M, Valerio C, Fusi V, Severgnini B, Lonati L, Magrini F, Zanchetti A. Cardiovascular target organ damage in essential hypertensives with or without reproducible nocturnal fall in blood pressure. J Hypertens. 2004;22:273–280. doi: 10.1097/00004872-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of the nighttime blood pressure. Hypertension. 2011;57:3–10. doi: 10.1161/HYPERTENSIONAHA.109.133900. [DOI] [PubMed] [Google Scholar]
- 19.Muntzel MS, Anderson EA, Johnson AK, Mark AL. Mechanisms of insulin action on sympathetic nerve activity. Clin Exp Hypertens. 1995;17:39–50. doi: 10.3109/10641969509087053. [DOI] [PubMed] [Google Scholar]
- 20.Reaven GM, Lithell H, Landsberg L. Hypertension and associated metabolic abnormalities – the role of insulin resistance and the sympathoadrenal system. N Engl J Med. 1996;334:374–381. doi: 10.1056/NEJM199602083340607. [DOI] [PubMed] [Google Scholar]
- 21.Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM, American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. doi: 10.1161/HYP.0000000000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron AD. Hemodynamic actions of insulin. Am J Physiol. 1994;267:187–202. doi: 10.1152/ajpendo.1994.267.2.E187. [DOI] [PubMed] [Google Scholar]
- 23.Creager MA, Liang CS, Coffman JD. Beta adrenergic-mediated vasodilator response to insulin in the human forearm. J Pharmacol Exp Ther. 1985;235:709–714. [PubMed] [Google Scholar]
- 24.Tamminem M, Westerbacka J, Vehkavaara S, Yki-Javinen H. Insulin-induced decreases in aortic wave reflection and central systolic pressure. Diabetes Care. 2002;25:2314–2319. doi: 10.2337/diacare.25.12.2314. [DOI] [PubMed] [Google Scholar]
- 25.Bloch CA, Clemons P, Sperling MA. Puberty decreases insulin sensitivity. J Pediatr. 1987;110:481–487. doi: 10.1016/s0022-3476(87)80522-x. [DOI] [PubMed] [Google Scholar]