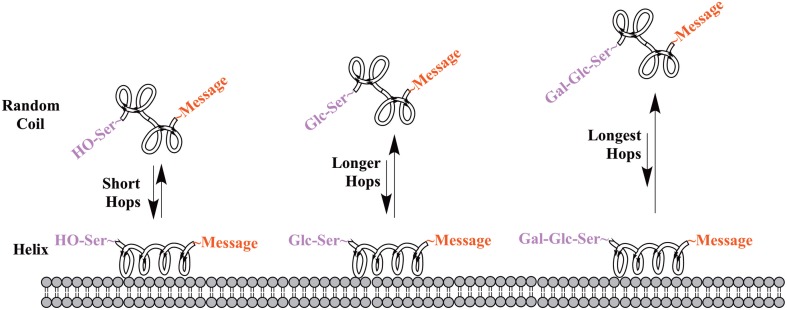
Figure 3.
Proposed mechanism of membrane “hopping.” In the CNS, a peptide will be present in equilibrium between two primary conformations: a random coil in aqueous environments, and a helix on a membrane surface. This equilibrium is governed by the peptide's ampipathicity: the proportion of the peptide that is hydrophilic vs. that which is hydrophobic. Theoretically, a glycopeptide will have a higher affinity for the aqueous environment than its non-glycosylated analog, leading to reduced time spent on the membrane surface. This is hypothesized to lead to longer “hops,” presumably allowing a glycopeptide to interrogate more of a membrane surface and find receptors.