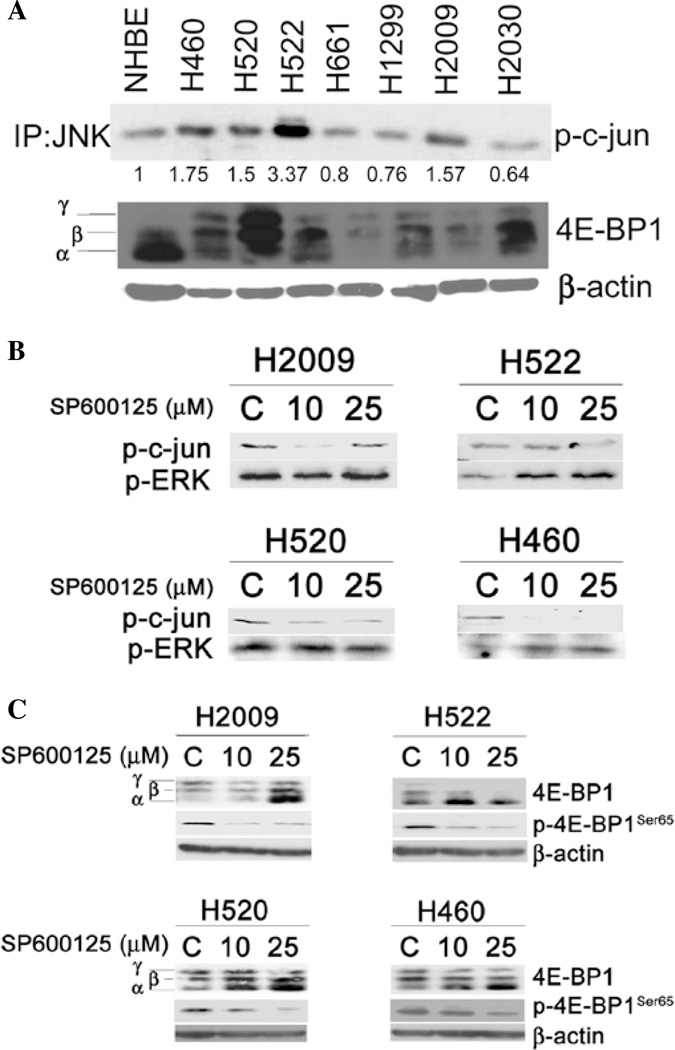
Figure 1.
JNK activity and effect of JNK inhibition on 4E-BP1 phosphorylation of NSCLC cells. (A) NSCLC cells and NHBE were grown under normal growing conditions. Lysates were subjected to in vitro kinase assay employing c-jun sepharose beads to immunoprecipitate JNK and membranes were then probed for phospho-c-jun. Numbers below the blot indicate fold-change in optical density when NHBE cells are used as the reference control. Also shown is a Western blot for 4E-BP1. β-actin was used as a loading control. (B) The indicated cell lines were treated with JNK inhibitor, SP600125, at the indicated concentrations for 24 h and subjected to in vitro kinase assay. JNK activity is indicated by its ability to phosphorylate c-junSer63. Western blotting for phospho-ERK1/2Thr202/Try204 are shown and β-actin is used as a loading control. (C) Cells treated with SP600125 and control were probed for 4E-BP1 by Western blotting. γ, β and α indicate hyper-, intermediate, and hypophosphorylated isoforms of 4E-BP1 respectively. Phospho-4E-BP1 was assayed using phospho-specific antibody for Ser65. β-actin was used as a loading control (C, vehicle control).