Figure 2.
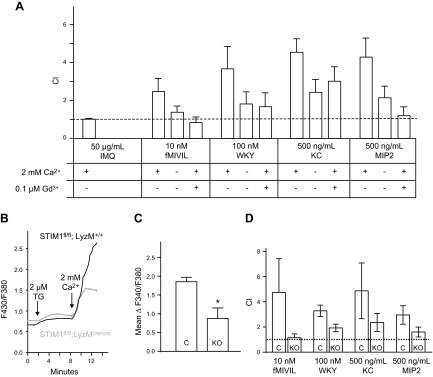
Effect of calcium removal and STIM1 KO on mouse neutrophil chemotaxis and SOCE. Neutrophil chemotaxis index was measured with a transwell assay. A) DiD-labeled neutrophils from mouse bone marrow migrate through a fibronectin-coated membrane (3 μm hole size) in response to 50 μg/ml IMQ, 10 nM fMIVIL, 100 nM WKYMVM, 500 ng/ml KC, or 500 ng/ml MIP-2 in the bottom well. Experiments were carried out in the presence or absence of 2 mM Ca2+ or in the presence of 2 mM Ca2+ and 0.1 μM gadolinium. Means ± sem from 3 independent experiments. B) Relative intracellular Ca2+ concentration in control and STIM1fl/fl KO mouse bone marrow neutrophils. Ca2+ stores were depleted with 2 μM thapsigargin (TG) in nominally Ca2+-free extracellular medium, and then 2 mM Ca2+ was restored 6 min later to reveal SOCE. Each trace represents the average response of 30 cells measured on a single coverslip. C) From experiments described in B, the average SOCE response above baseline (mean ΔF340/380 ± sem) from 3 coverslips. *Significant difference between cells from control (C) and KO mice (P < 0.05) based on a Student t test. For B and C, Rmax was 3.3. D) DiD-labeled cell transwell chemotaxis assay as described in A. Chemotaxis index of blood neutrophils from control (C) or STIM1fl/fl mice (KO) in response to 10 nM fMIVIL, 100 nM WKYMVM, 500 ng/ml KC, or 500 ng/ml MIP-2 placed in the bottom well. Chemotaxis index was recorded in presence of 2 mM Ca2+. Scale bar, sem from 3 independent experiments.
