Abstract
Glycosaminoglycan (GAG) polysaccharides have been implicated in a variety of cellular processes, and alterations in their amount and structure have been associated with diseases such as cancer. In this study, we probed 11 sugar analogs for their capacity to interfere with GAG biosynthesis. One analog, with a modification not directly involved in the glycosidic bond formation, 6F-N-acetyl-d-galactosamine (GalNAc) (Ac3), was selected for further study on its metabolic and biologic effect. Treatment of human ovarian carcinoma cells with 50 μM 6F-GalNAc (Ac3) inhibited biosynthesis of GAGs (chondroitin/dermatan sulfate by ∼50–60%, heparan sulfate by ∼35%), N-acetyl-d-glucosamine (GlcNAc)/GalNAc containing glycans recognized by the lectins Datura stramonium and peanut agglutinin (by ∼74 and ∼43%, respectively), and O-GlcNAc protein modification. With respect to function, 6F-GalNAc (Ac3) treatment inhibited growth factor signaling and reduced in vivo angiogenesis by ∼33%. Although the analog was readily transformed in cells into the uridine 5′-diphosphate (UDP)-activated form, it was not incorporated into GAGs. Rather, it strongly reduced cellular UDP-GalNAc and UDP-GlcNAc pools. Together with data from the literature, these findings indicate that nucleotide sugar depletion without incorporation is a common mechanism of sugar analogs for inhibiting GAG/glycan biosynthesis.—Van Wijk, X. M., Lawrence, R., Thijssen, V. L., van den Broek, S. A., Troost, R., van Scherpenzeel, M., Naidu, N., Oosterhof, A., Griffioen, A. W., Lefeber, D. J., van Delft, F. L., van Kuppevelt, T. H. A common sugar-nucleotide-mediated mechanism of inhibition of (glycosamino)glycan biosynthesis, as evidenced by 6F-GalNAc (Ac3).
Keywords: sugar analog, angiogenesis, growth factor signaling, glycobiology
Introduction
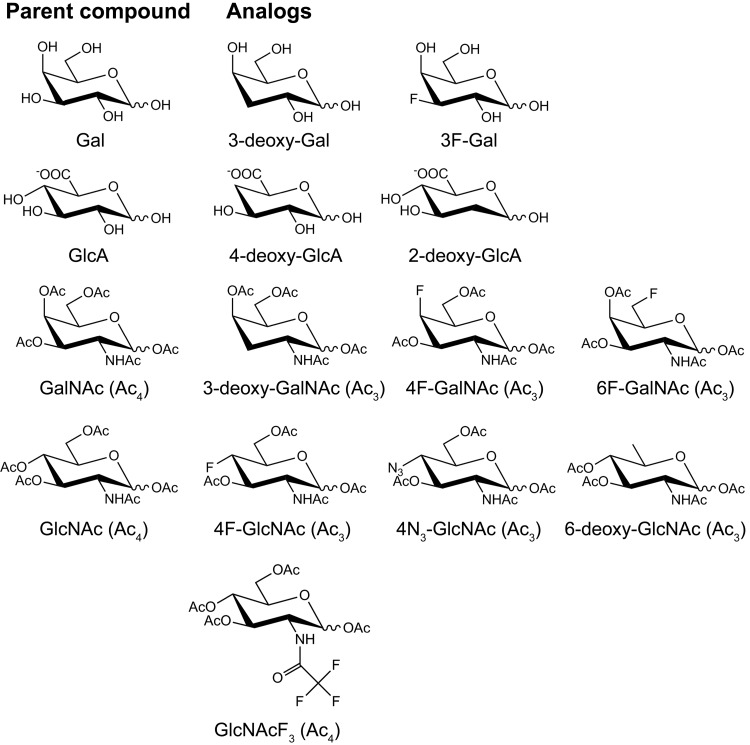
Glycosaminoglycans (GAGs) as chondroitin/dermatan sulfate (CS/DS) and heparan sulfate (HS) are long, linear, strongly negatively charged polysaccharide chains that are linked covalently to a protein core. They are present at the cell surface of virtually every mammalian cell and in the extracellular matrix. GAGs are important in a wide variety of physiological processes by interacting with numerous effector molecules (e.g., growth factors and their receptors) (1). GAGs are composed of repeating disaccharides of d-glucuronic acid (GlcA) and either N-acetyl-d-galactosamine (GalNAc) in CS/DS ([-4GlcA-β1-3GalNAc-β1-]n) or N-acetyl-d-glucosamine (GlcNAc) in HS ([-4GlcA-β1-4GlcNAc-α1-]n). Variable modification of the resulting polysaccharides occurs by epimerization of GlcA to l-iduronic acid (IdoA) and various sulfation reactions (2). The quantity and fine structure of GAGs are often changed under pathological conditions, including cancer (3). For example, 6-O-sulfated CS disaccharides are increased up to 50-fold in gastrointestinal carcinoma (4), and 6-O-sulfated and 4-O-, 6-O-disulfated CS structures are elevated in ovarian cancer (5, 6). Furthermore, HS 6-O sulfation is of significant importance in fibroblast growth factor (FGF)-2/vascular endothelial growth factor (VEGF) signaling (7) and (tumor) angiogenesis (8), and silencing of HS 6-O-sulfotransferase-2 inhibits angiogenesis and tumorigenesis in mice (9, 10). These effects underscore the need for compounds that can interfere with GAG function, biosynthesis, and specific sulfation. The use of sugar analogs, which are chemically modified monosaccharides, has been a viable approach for altering the biosynthesis of GAGs and other glycans in vitro and in vivo (11–21). In this study, we tested 11 sugar analogs (Fig. 1) for their capacity to interfere with GAG chain elongation or GAG sulfation and further focused on peracetylated 6-fluoro-GalNAc [6F-GalNAc (Ac3)]. This sugar analog is modified at a position that is not directly involved in glycosidic bond formation and can potentially inhibit GAG 6-O sulfation.
Figure 1.
Structure of the (peracetylated) sugar analogs and their parent (peracetylated) sugars. The 3-deoxy- and 3F-Gal analogs were anticipated to inhibit HS and CS/DS synthesis, in that these GAGs contain a GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser linkage tetrasaccharide. Analogs of GlcA were synthesized, as GlcA is common to GAGs and is not abundantly present in other glycan structures. 4-Deoxy-GlcA was anticipated to inhibit CS/DS and HS chain elongation, whereas 2-deoxy-GlcA was anticipated to inhibit 2-O sulfation of these GAGs. The 3-deoxy-, 4F-, and 6F-GalNAc (Ac3) analogs were anticipated to inhibit CS/DS chain elongation, CS/DS 4-O sulfation, and CS/DS 6-O sulfation, respectively. The 4F-, 4N3-, and 6-deoxy-GlcNAc (Ac3) and GlcNAcF3 (Ac4) analogs were anticipated to inhibit HS chain elongation [both 4F- and 4N3-GlcNAc (Ac3)], HS 6-O sulfation, and HS N sulfation, respectively.
MATERIALS AND METHODS
Monosaccharides
2-Deoxy-GlcA, 4-deoxy-GlcA, 3-deoxy-GalNAc (Ac3), 4N3-GlcNAc (Ac3), and 6-deoxy-GlcNAc (Ac3) were synthesized as described in the Supplemental Methods and Fig. 2. GlcNAc (Ac4) was synthesized as described previously (20). Peracetylated 4F-GalNAc (2-acetamido-1,3,6-tri-O-acetyl-2,6-dideoxy-4-fluoro-d-galactopyranose) and peracetylated 6F-GalNAc (2-acetamido-1,3,4-tri-O-acetyl-2,6-dideoxy-6-fluoro-d-galactopyranose) were purchased from Sussex Research (Ottawa, ON, Canada). Structures were confirmed by 1H and 19F NMR and mass spectrometry (MS). Purity for 6F-GalNAc was >95%, as determined by HPLC. A stock solution of 100 mM in ultrapure water was prepared. The purity of 4F-GalNAc was >90%, as estimated by 1H NMR. A stock solution of 100 mM in 75% DMSO was prepared. Peracetylated GalNAc (2-acetamido-1,3,4,6-tetra-O-acetyl-2- d-galactopyranose) was purchased from Carbosynth (Berkshire, United Kingdom). Purity was 98.1%, and structure was confirmed by NMR. Stock solutions of 100 mM in DMSO and 25 mM in ultrapure water were prepared as a control for peracetylated 4F- and 6F-GalNAc, respectively. Peracetylated 4F-GlcNAc (2-acetamido-1,3,6-tri-O-acetyl-2,4-dideoxy-4-fluoro-d-glucopyranose) was purchased from Carbosynth. Purity was 98%, as determined by TLC, and 1H NMR conformed to structure. A stock solution of 100 mM in 75% DMSO was prepared. 3-Deoxy-d-galactose and 3F-Gal (3-deoxy-3-fluoro-d-galactose) were purchased from Carbosynth. The purity of 3-deoxy- d-galactose was >95% and 1H NMR conformed to structure. The purity of 3F-Gal was 99.9%, as determined by HPLC, and 13C NMR conformed to structure. Peracetylated GlcNAcF3 (1,3,4,6-tetra-O-acetyl-2-deoxy-2-trifluoroacetamido-d-glucose) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Purity was >90%. A stock solution of 100 mM in 75% DMSO was prepared.
Figure 2.
Synthesis of monosaccharides: A) 6-Deoxy-GlcNAc (Ac3) (compound 7; 1,3,4-tri-O-acetyl-2,6-dideoxy-2-N-acetyl-glucosamine); B) 3-deoxy-GalNAc (Ac3) (compound 6; 1,4,6-tri-O-acetyl-2,3-dideoxy-2-N-acetyl-galactosamine); C) 4N3-GlcNAc (Ac3) (compound 6; 1,3,6-tri-O-acetyl-2,4-dideoxy-2-N-acetyl-4-azido-glucosamine); D) 4-deoxy-glucuronic acid (compound 5); and E) 2-deoxy-glucuronic acid (compound 5). AIBN, azobisisobutyronitrile; DBU, 1,8-diazabicyclo[5.4.0]undec-7-ene; DCM, dichloromethane; DIPEA, di-isopropylethylamine; DMAP, 4-dimethylaminopyridine; DMF, dimethylformamide; TBAI, tetrabutyl ammonium iodide; TCDI, 1,1′-thiocarbonyldiimidazole; TEMPO, 2,2,6,6-tetramethylpiperidinyloxy; TESOTf, triethylsilyl trifluoromethanesulfonate.
Cell culture
SKOV3 ovarian carcinoma cells, MV3 melanoma cells, HeLa cervical cancer cells, human umbilical vein endothelial cells (HUVECs), and RF24 endothelial cells were cultured as described previously (16, 20). The cells were seeded 1 day before the addition of the sugar (analog). After 3 days of culturing in the presence of one of these sugars, the cells were used for further analyses.
Flow cytometry and immunocytochemistry
Flow cytometry and immunocytochemistry were performed as described previously (16, 20). The single-chain variable fragment antibody GD3A12 was used to analyze DS (22). To determine the binding capacity of FGF-2, SKOV3 cells were incubated with 1 µg/ml recombinant human (rh)FGF-2 (R&D Systems, Minneapolis, MN, USA) and this was detected as described previously (20).
Proliferation assays
SKOV3 and HUVEC proliferation was assessed as reported earlier (20). In brief, SKOV3 cells were incubated for 3 days with various concentrations of the sugar (analog), and the amount of metabolic active cells was assessed by the WST-1 assay (Roche Diagnostics, Almere, The Netherlands). Primary HUVECs were incubated for 3 days with medium containing 1% human serum and 1% fetal bovine serum, GalNAc (Ac4) or 6F-GalNAc (Ac3) in various concentrations, and 10 ng/ml FGF-2 and assayed by the CellTiter-Glo assay (Promega, Madison, WI, USA).
GAG isolation and agarose gel electrophoresis
GAGs were isolated, separated, and stained on agarose gel as described previously (23). In short, GAGs were isolated by papain digestion, TCA precipitation, and ion exchange on Vivapure Q Mini H spin columns (Sartorius AG, Goettingen, Germany) and by methanol precipitation. The GAGs were separated on 1% agarose in 50 mM Ba(Ac)2 buffer and stained with a combined azure A-silver staining procedure.
HPLC disaccharide analysis
For experiments with 6F-GalNAc (Ac3), disaccharide analysis was performed as described (20). In short, CS/DS or HS disaccharides were obtained by digestion with chondroitinase ABC or by a combination of heparinase I, II, and III, respectively, and separation was performed by ion pair reverse-phase (RP) chromatography with a gradient elution on a Supelcosil LC-18-T column (Sigma-Aldrich Chemie BV, Zwijndrecht, The Netherlands). The identity of the disaccharides was determined by disaccharide standards, and the amount of specific disaccharides was calculated by peak surface area analysis. For experiments with 4F-GalNAc (Ac3) and 4F-GlcNAc (Ac3), CS/DS disaccharides were obtained by digestion with chondroitinase ABC, labeled with 2-aminoacridone (AMAC), and separated and detected by RP-HPLC, as described previously (24).
Glycan reductive isotope labeling-liquid chromatography/MS analysis of GAG disaccharides and uridine 5′-diphosphate hexosamines
Analysis of CS/DS and HS disaccharides and uridine 5′-diphosphate (UDP) hexosamines was assessed as reported previously (20, 25). Samples were analyzed by liquid chromatography-MS (LC/MS) on an LTQ Orbitrap Discovery electrospray ionization mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) equipped with an Ultimate 3000 quaternary high-performance liquid chromatography micropump (Dionex, Sunnyvale, CA, USA) and a C-18 RP microbore column (1 × 150 mm, 5 μm; Higgins Analytical, Mountain View, CA, USA).
Nano-LC-Chip-quadrupole time-of-flight monosaccharide analysis
Isolated GAGs from SKOV3 cells, cultured with or without 50 µM 6F-GalNAc (Ac3), were dissolved in 100 µl ultrapure water. To isolate a comparable amount of GAGs, GAGs from untreated cells and treated cells were derived from 3 and 6 confluent culture flasks (175 cm2), respectively. The GAGs were hydrolyzed by the addition of 400 µl 6 M HCl and incubated at 105°C for 6 h under nitrogen gas in sealed glass tubes. Ten microliters of a 100 mM 6F-GalNAc (Ac3) solution was hydrolyzed as a control. HCl and water were removed by drying the samples in a vacuum desiccator in the presence of NaOH pellets. The samples were dissolved in 1 ml ultrapure water, lyophilized, and dissolved in 50 µl ultrapure water. To improve ionization, 10 µl of a 50% MeOH and 0.1% formic acid solution (v/v/v) in ultrapure water was added to 20 µl of each sample. Analysis was performed in positive mode with a microfluidic 6540 HPLC-chip-quadrupole time-of-flight instrument (Agilent Technologies Netherlands BV, Middelburg, The Netherlands) by direct infusion via a syringe pump and a direct-infusion chip with a nanoelectrospray tip. Drying gas was set at a flow rate of 3.0 L/min, and a temperature of 300°C; capillary voltage was set at 1900 V and fragmentor voltage at 175 V. Data analysis was performed with Agilent Mass Hunter Qualitative Analysis Software B.04.00.
High-performance anion-exchange chromatography-UV analysis of (sugar) nucleotides
Analysis of sugar nucleotides was performed by the UCSD Glycotechnology Core (University of San Diego, La Jolla, CA, USA), as described previously (20). In brief, cells were pelleted and lysed by sonication, and ethanol was added to the supernatant to 80% (v/v). After centrifugation, the supernatant was dried under nitrogen and dissolved in water. Separation of different (sugar) nucleotides was carried out by Dx600 high-performance anion exchange chromatography (HPAEC)-UV on a Dionex Analytical CarboPac PA 1 column.
Detection of phosphorylated ERK1/2- and O-GlcNAc-modified proteins
Detection of phospho-ERK1/2- and O-GlcNAc-modified proteins was performed as described (20). In brief, HUVECs, treated with or without 50 µM GalNAc (Ac4) or 6F-GalNAc (Ac3) for 3 d, were incubated with medium containing 0.25% newborn calf serum and 0.25% human serum for 6 hours, after which 10 ng/ml rhFGF-2 or rhVEGF (R&D Systems) was added, and the HUVECs were harvested 10 min later. For analysis of O-GlcNAc, SKOV3 cells were cultured for 3 d, with or without 50 µM GalNAc (Ac4), 4F-GlcNAc (Ac3), 4F-GalNAc (Ac3), or 6F-GalNAc (Ac3), and harvested in sample buffer.
Chorioallantoic membrane assay
The chicken chorioallantoic membrane (CAM) assay was performed as described previously (20). In brief, treatment consisted of daily application of GalNAc (Ac4) or 6F-GalNAc (Ac3) at the indicated concentrations in a volume of 50 µl within a nonsilicone ring (intraoral elastic band; diameter, 9 mm) applied on the CAM for 4 days. Vessel density was quantified as the total number of cross sections of the blood vessels with 5 concentric circles projected onto a photograph of the CAM. Experiments were performed in accordance with the legal requirements of the local ethics committee.
Statistics
Data are presented as the mean with sd, unless indicated otherwise. Data from the disaccharide analysis, lectin flow cytometry, and HUVEC and SKOV3 proliferation were analyzed with a 2-tailed Student’s t test, with significance set at P < 0.05. CAM assay data were analyzed with the Mann-Whitney U test.
RESULTS
4F- and 6F-modified sugar analogs reduce expression of GAGs and other glycans
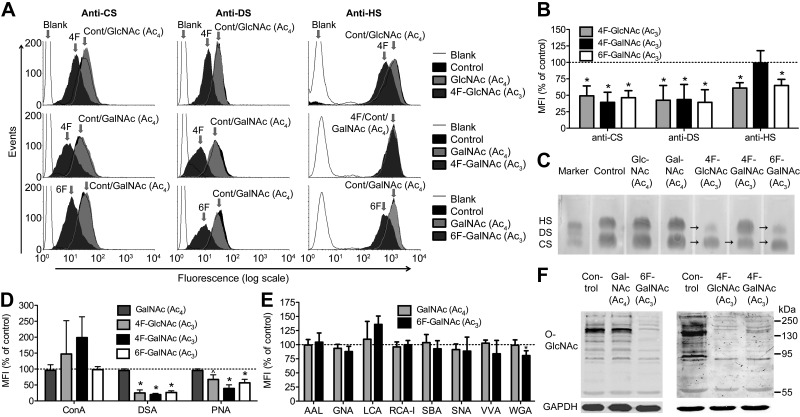
We evaluated the sugar analogs of 4 different classes: galactose, a component of the tetrasaccharide region that links CS/DS/HS to the core protein; GlcA, a component of both CS/DS and HS disaccharides; GlcNAc, a component of HS disaccharides; and GalNAc, a component of CS/DS disaccharides (Fig. 1). We screened the inhibitory capacity of these analogs on the biosynthesis of GAGs and other glycans by culturing human ovarian carcinoma cells (SKOV3) for 3 days in the presence of a sugar analog. Cell-surface binding of anti-GAG antibodies and lectins recognizing N- and O-linked glycans was measured by flow cytometry. Three peracetylated sugar analogs, 4F-GlcNAc, 4F-GalNAc, and 6F-GalNAc, but not their parent peracetylated sugars GlcNAc and GalNAc, were found to reduce anti-GAG antibody binding at 50 µM (Fig. 3A, B). At this concentration, cellular proliferation was not inhibited by 4F-GalNAc (Ac3) and 6F-GalNAc (Ac3) and only to a small extent (∼14%) by 4F-GlcNAc (Ac3) (Supplemental Fig. S1). The total amount of GAGs was also reduced, as shown by agarose gel electrophoresis (Fig. 3C). The 3 sugar analogs affected GAG biosynthesis to a similar extent, except for HS biosynthesis, which was not affected by 4F-GalNAc (Ac3) (Fig. 3A–C). In addition to GAGs, these analogs affected N- and O-linked glycan biosynthesis in a similar pattern (Fig. 3D). Expression of poly-N-acetyllactosamine ([-3Gal-β1-4GlcNAc-β1-]n), as recognized by Datura stramonium agglutinin (DSA), was most strongly inhibited, followed by the core 1 O-GalNAc glycans, as recognized by peanut agglutinin (PNA), whereas expression of mannose-rich N-linked glycans, as recognized by concanavalin (ConA) lectin, was largely unaffected. For 6F-GalNAc (Ac3), staining of an additional 8 lectins was evaluated, and there was no reduction in staining by lectins that recognize mannose [Galanthus nivalis agglutinin; (GNA)], fucose [Aleuria aurantia lectin (AAL) and Lens culinaris agglutinin (LCA)], or sialic acid [(Sambucus nigra agglutinin (SNA)] (Fig. 3E), indicating that predominantly GlcNAc/GalNAc-containing glycans had been affected. The remaining sugar analogs (8 of 11) at various concentrations did not obviously affect biosynthesis of GAGs and other glycans, as evaluated by flow cytometry, immunocytochemistry, and agarose gel electrophoresis (Supplemental Fig. S2). These analogs are covered further in the Discussion. Next, we evaluated the effect of 4F-GlcNAc (Ac3), 4F-GalNAc (Ac3), and 6F-GalNAc (Ac3) on O-GlcNAc protein modification (Fig. 3F). All 3 sugar analogs inhibited protein O-GlcNAc levels, whereas the parent acetylated sugars had no effect [Fig. 3F, for GlcNAc (Ac4) see (20)]. The activity of the responsible transferase for this modification (O-GlcNAc transferase) is dependent on the cellular UDP-GlcNAc concentration over a large concentration range (26), suggesting that UDP-GlcNAc levels have been reduced by the sugar analog treatment. Taken together, these results are in good agreement with earlier data for 4F-GlcNAc (Ac3) and 4F-GalNAc (Ac3), showing inhibition of biosynthesis of poly-N-acetyllactosamine (27–29) and of the GAGs for 4F-GlcNAc (Ac3) (11, 15). In addition, it has been shown that treatment of cells with 4F-GlcNAc (Ac3) reduces levels of UDP-GlcNAc (28, 30). We decided to focus further on 6F-GalNAc (Ac3), a sugar analog that has not been tested on cells before and that is modified at a position not directly involved in glycosidic bond formation or in epimerization of UDP-GalNAc to UDP-GlcNAc and vice versa.
Figure 3.
4F- and 6F-modified sugar analogs interfere with biosynthesis of GAGs and other glycans. A) Flow cytometry analysis of GAG expression of SKOV3 cells, treated with 50 µM sugar analog or the parent peracetylated sugar for 3 days, with 3 anti-GAG antibodies (CS: antibody IO3H10; DS: antibody GD3A12; and HS: antibody HS4C3). B) Quantification of the peracetylated sugar analogs as shown in (A). C) Agarose gel electrophoresis of isolated GAGs from SKOV3 cells cultured for 3 days, with or without a 50 µM concentration of the indicated sugar analog. Note the reduction (arrows) in CS and HS in the case of 4F-GlcNAc (Ac3) and 6F-GalNAc (Ac3), and CS in the case of 4F-GalNAc (Ac3). D) Flow cytometric analysis for N- and O-linked glycan expression of SKOV3 cells using 3 lectins (ConA: oligomannose, hybrid, and biantennary complex N-glycans; DSA: poly-N-acetyllactosamine; and PNA: Gal-β1-3GalNAcα-R found on O-glycans). E) Flow cytometric analysis for N- and O-linked glycan expression of SKOV3 cells treated with 50 µM GalNAc (Ac4) or 50 µM 6F-GalNAc (Ac3), with 8 additional lectins (AAL: α1-2/3/6 linked Fuc to Gal/GlcNAc found on N-glycans; GNA: terminal α1-3Man found on oligomannose N-glycans; LCA: fucosylated core region of bi- and triantennary complex N-glycans; RCA-I: terminal βGal found on N-glycans; SBA: GalNAcα/β-R found on O-glycans; SNA: Siaα2-6Gal(NAc)-R; VVA: GalNAcα-R found on O-glycans; and WGA: GlcNAcβ-R, Neu5Acα-R found on N- and O-glycans). F) Analysis of O-GlcNAc protein modification, as determined by Western blot with an antibody against this modification and GAPDH (control). MFI, mean fluorescence intensity; RCA-I, Ricinus communis agglutinin-I; SBA, soybean agglutinin; VVA, Vicia villosa agglutinin; WGA, wheat germ agglutinin. Data are expressed as the means ± sd. *P < 0.05, ^P = 0.06, compared with the untreated control. Student’s t test (n = 3 or more).
6F-GalNAc is not incorporated into GAGs and reduces UDP-GlcNAc and UDP-GalNAc levels
To exclude that the observed effects of 6F-GalNAc (Ac3) are restricted to SKOV3 cells, endothelial (RF24) and cervical cancer (HeLa) cells were treated and assayed for cell surface GAG levels (Supplemental Fig. S3). For RF24 cells, the effect of 50 µM 6F-GalNAc (Ac3) on CS and HS expression was comparable to that of SKOV3 cells. For HeLa cells, 300 µM was necessary for a similar effect, indicating cell-specific sensitivity. To test whether 6F-GalNAc (Ac3) could serve as a potential inhibitor of GAG 6-O sulfation, we evaluated incorporation of 6F-GalNAc into GAGs by MS. Natural hexosamines (GlcN and GalN), obtained after 6 M HCl hydrolysis, were readily found in GAGs isolated from untreated and 6F-GalNAc (Ac3)-treated SKOV3 cells, but 6F-hexosamines were not detected (Supplemental Fig. S4). 6F-GalNAc was also not found in GAG disaccharides after chain depolymerization with heparinases and chondroitinase ABC (Supplemental Table S1). In addition, we did not detect 6F-containing mono- or trisaccharides originating from the chain terminus [the nonreducing end (NRE)]. These structures could be present after enzymatic depolymerization if 6F-GalNAc incorporation leads to chain termination by not acting as an acceptor for the polymerizing enzymes. Natural NRE disaccharides were detected in GAG chains from 6F-GalNAc (Ac3)-treated cells in an increased ratio relative to the corresponding internal disaccharides, compared to the untreated control (Table 1). This finding is consistent with a reduction in chain size. A similar observation was made with 4F-GlcNAc (Ac3). GAG disaccharide composition analysis further revealed an increase in HS 6-O sulfation (Supplemental Fig. S5B–D, F), similar to the effect of 4F-GlcNAc (Ac3) (Supplemental Fig. S5D, F) and another sugar analog, 4-deoxy-GlcNAc (Ac3) (20), but a reduction in the uronic acid (UA)-GalNAc-6S CS/DS disaccharide (Supplemental Fig. S5A), similar to the effect of 4F-GlcNAc (Ac3) and 4F-GalNAc (Ac3) (Supplemental Fig. S5E). These changes are possibly explained by shortening of the GAG chains, as there is evidence that GAG sulfation patterns are nonrandomly distributed throughout the chain (31–33).
TABLE 1.
Ratio of NRE disaccharides to internal disaccharides
| Disaccharidea | NRE massb (m/z) | Internal mass (m/z) | Intensity ratio (NRE/internal) (%) |
|||
|---|---|---|---|---|---|---|
| Control (%) | 4F-GlcNAc (Ac3)(%) | 6F-GalNAc (Ac3)(%) | ||||
| HS: UA-GlcNS+1S | 591.08 | 573.07 | 1.9 | 10.8 | 10.8 | |
| CS/DS: UA-GalNAc+1S | 553.14 | 535.13 | 2.0 | 6.2 | 6.5 | |
GAGs of SKOV3 cells cultured with 50 µM 6F-GalNAc (Ac3) or 4F-GlcNAc (Ac3) were depolymerized by chondroitinase ABC for CS/DS analysis or by a combination of heparinases I, II, and III for HS analysis. Samples were analyzed by LC/MS on an LTQ Orbitrap Discovery system (Thermo Fisher Scientific) equipped with a C-18 RP microbore column. a1S indicates that 1 SO3− group is present. In the case of HS, the most likely structures are UA2S-GlcNS and UA-GlcNS6S. For CS/DS, the most likely structures are UA-GalNAc4S and UA-GalNAc6S.bThe mass of the NRE disaccharide is +18 amu over the corresponding internal disaccharide, because of the elimination of an H2O molecule from the internal disaccharide by heparinase.
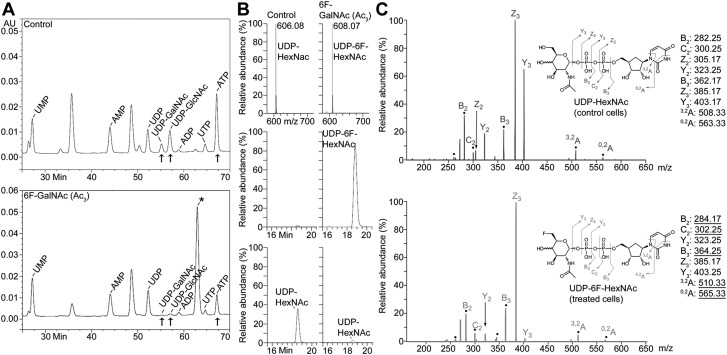
Next, we evaluated sugar nucleotide levels. Levels of UDP-GlcNAc and -GalNAc were strongly reduced by treatment with 6F-GalNAc (Ac3), as evaluated by HPAEC-UV and LC/MS (Fig. 4A, B, bottom), likely explaining the effect on biosynthesis of GAGs and other glycans. HPAEC-UV analysis also revealed an unidentified sugar nucleotide abundantly present in 6F-GalNAc (Ac3)-treated, but not untreated, cells (Fig. 4A), corresponding to a UDP-activated product of 6F-GalNAc—UDP-6F-GalNAc and UDP-6F-GlcNAc [UDP-6F-N-acetyl-hexosamine (HexNAc)]. Indeed, MS showed that UDP-6F-HexNAc, with a mass increased by 2 atomic mass units (amu), compared with natural UDP-HexNAc (UDP-GlcNAc and -GalNAc) (Fig. 4B, top), was abundantly present (Fig. 4B, middle). Fragmentation by collision-induced dissociation confirmed the sugar nucleotide’s identity, as mass-to-charge (m/z) ratios corresponding to UDP and monosaccharide fragments were found (Fig. 4C). As expected, only the NRE fragment ions that include carbon-6 differed by 2 amu, underlined m/z values to the right). These data indicate that, although 6F-GalNAc is not incorporated, it enters the cells, is deacetylated, by aspecific esterases (34), and is transformed into UDP-6F-GalNAc by enzymes of the salvage pathway (GalNAc kinase and UDP-GalNAc pyrophosphorylase). The phosphorylation of 6F-GalNAc may be surprising, given that human recombinant GalNAc kinase, involved in phosphorylation of GalNAc, does not tolerate a deoxy modification at C6 (35). Although we have not specifically addressed the question of where 6F-GalNAc (Ac3) ends up, from the analyses shown in Fig. 4, it is likely that a large amount eventually becomes UDP-6F-HexNAc. We also noted a decreased ATP level (Fig. 4A), possibly due to extensive sugar analog UDP activation.
Figure 4.
6F-GalNAc (Ac3) is UDP activated and reduces cellular UDP-GlcNAc and -GalNAc. A) HPAEC-UV analysis of sugar nucleotides. An additional (sugar) nucleotide (*), most likely representing UDP-6F-HexNAc (see B), is present in 50 µM 6F-GalNAc (Ac3)-treated SKOV3 cells. Treatment of SKOV3 cells with 6F-GalNAc (Ac3) reduces UDP-GlcNAc, UDP-GalNAc, and ATP. The amount of 6F-GalNAc (Ac3)-treated cells used for extraction was 86% of the control. B) LC/MS analysis of sugar nucleotides. The mass of UDP-6F-HexNAc was increased by 2 amu, compared with UDP-HexNAc (top). The extracted ion current shows that UDP-6F-HexNAc was abundantly present after 50 µM 6F-GalNAc (Ac3) treatment (middle), whereas UDP-HexNAc (bottom) was strongly reduced. The amount of 6F-GalNAc (Ac3)-treated cells used for extraction was 79% of the control. C) Tandem MS with constellation of daughter ions of sugar nucleotides. UDP-HexNAc from control (untreated) cells (top) and UDP-6F-HexNAc from 6F-GalNAc (Ac3)-treated cells (bottom) were analyzed. Assignments and their m/z values are indicated by the molecular diagram (right). Ions marked with a dot represent NRE fragment ions that include carbon-6, which differs by 2 amu, depending on whether the parent molecule is HexNAc or 6F-HexNAc. A–C) The experiments shown were conducted together with those in untreated cells, for which the data have been published (20). MFI, mean fluorescence intensity; UMP, uridine 5′-monophosphate; UTP, uridine 5′-triphosphate.
6F-GalNAc reduces FGF-2/VEGF signaling and angiogenesis
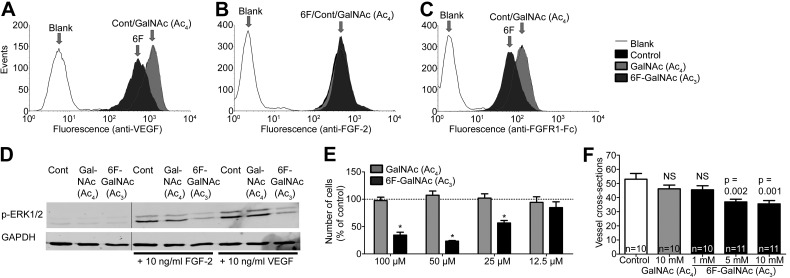
We recently reported on the effects of another sugar analog, 4-deoxy-GlcNAc (Ac3) (20). Overall, results of this analog were very similar to the effects of 6F-GalNAc (Ac3) described here. 4-Deoxy-GlcNAc (Ac3) was activated into UDP-4-deoxy-GlcNAc, was not incorporated, reduced UDP-GlcNAc and -GalNAc levels, and reduced biosynthesis of GAGs and other glycans, including poly-N-acetyllactosamine and O-GlcNAc protein modification (20). In addition, exon array analysis revealed reduced expression of genes regulated by the proangiogenic HS-binding growth factors VEGF and FGF-2. The cellular binding and signaling of these factors was inhibited, as was angiogenesis in vivo (20). We decided to evaluate the biologic effects of 6F-GalNAc (Ac3) on growth factor signaling and angiogenesis, similar to 4-deoxy-GlcNAc (Ac3). Treatment with 6F-GalNAc (Ac3) reduced cell surface binding of VEGF and fibroblast growth factor receptor (FGFR)-1, but not of FGF-2, to SKOV3 cells (Fig. 5A–C), and signal transduction, as analyzed by phospho-ERK in primary endothelial cells (HUVECs) stimulated with FGF-2 or VEGF (Fig. 5D). Although 6F-GalNAc (Ac3) did not reduce cell surface FGF-2 binding, the reduced binding of (the ectodomain of) its receptor FGFR1 suggests a reduced ternary complex formation (FGF2-FGFR1-HS), consistent with reduced FGF-2-stimulated pERK signaling. Furthermore, FGF-2-stimulated HUVEC proliferation was dose-dependently inhibited (Fig. 5E). To test for inhibition of the formation of new blood vessels in vivo, we used the CAM assay. Daily treatment with 50 µl of 5 or 10 mM 6F-GalNAc (Ac3) significantly reduced vessel density (∼30%), whereas GalNAc (Ac4) had no significant effect (Fig. 5F). Thus, not only were the metabolic effects of 6F-GalNAc (Ac3) similar to those reported for 4-deoxy-GlcNAc (Ac3), but also the biologic effects on growth factor signaling and angiogenesis.
Figure 5.
6F-GalNAc (Ac3) inhibits FGF-2 and VEGF signaling and reduces in vivo angiogenesis. Treatment of SKOV3 cells with 6F-GalNAc (Ac3), but not GalNAc (Ac4), reduces (A) recombinant rat VEGF164, but not (B) rhFGF-2, binding, as determined by addition of growth factor and flow cytometry with an antibody against VEGF or FGF-2. C) 6F-GalNAc (Ac3) reduces binding of (soluble) recombinant human FGFR1 (Fc chimera) to SKOV3 cells, previously incubated with rhFGF2 and assayed with an anti-IgG antibody. D) 6F-GalNAc (Ac3), but not GalNAc (Ac4), inhibits formation of intracellular phospho-ERK1/2 (essential in downstream signaling by FGF-2 and VEGF) in HUVECs, as determined by Western blot with an antibody against phospho-ERK 1/2 or GAPDH (control). One lane containing the molecular weight standard was removed between lanes 3 and 4, as indicated by the vertical line. E) 6F-GalNAc (Ac3), but not GalNAc (Ac4), inhibits FGF-2 (10 ng/ml)-induced proliferation of HUVECs, as determined by the CellTiter-Glo assay (Promega). Data are expressed as the means ± sd of 3 separate experiments. *P < 0.05 compared to untreated cells. Student’s t test. F) 6F-GalNAc (Ac3), but not GalNAc (Ac4), inhibits in vivo angiogenesis (vessel density) in the CAM assay. Data are expressed as the means ± sem. Probabilities are as indicated, determined by the Mann-Whitney U test.
DISCUSSION
Taken together, the data for 6F-GalNAc (Ac3) are in line with data in the literature for 2 other peracetylated sugar analogs that inhibit GAG biosynthesis: 4-deoxy-GlcNAc (20) and 4F-GlcNAc (15, 28, 30). Similarly, abundant transformation of the analog into its UDP-sugar was observed, and parent natural UDP-sugar levels were reduced. However, these 2 analogs are modified at a position that is directly involved in glycosidic bond formation in HS, and incorporation would result in chain termination. MS analysis showed that 4-deoxy-GlcNAc (Ac3) was not incorporated into HS (20), but lack of incorporation of 4F-GlcNAc (Ac3) has been shown only for N-linked glycans (28). In addition, the C4-OH position is directly involved in epimerization of UDP-GalNAc into UDP-GlcNAc and vice versa, and these analogs may inhibit epimerase activity, as suggested for UDP-4F-GlcNAc (15). In contrast, 6F-GalNAc (Ac3) is modified at a position that is not directly involved in either of these processes, and the comparable effect of 4-deoxy-GlcNAc (Ac3), 4F-GlcNAc (Ac3), 4F-GalNAc (Ac3), and 6F-GalNAc (Ac3) on biosynthesis of GAGs and other glycans suggests a common mechanism of UDP-sugar depletion without incorporation (Fig. 6). This line of thinking is supported by data for sugar analogs that affect glycans other than GAGs, including 5T-Fuc (36), 2F-Fuc (18), and 3Fax-NeuAc (18). These analogs were also transformed into their nucleotide sugars, but they were not incorporated, the parent natural nucleotide sugar levels were reduced, and glycan biosynthesis was inhibited. Reduced natural nucleotide sugar levels may result from abundant activation of the sugar analog by the same sugar activation machinery that is used by the natural sugar. In addition, accumulation of a nucleotide-activated sugar analog inside the cell may inhibit the de novo synthesis of natural nucleotide sugars as a result of a feedback mechanism, as proposed for 2F-Fuc and 3Fax-NeuAc (18). Nucleotide-activated sugar analogs may also inhibit glycosyltransferases (17, 18), although we did not evaluate this possibility. Although similar overall, sugar analogs have specific differences. For example, it is currently not clear why 4F-GalNAc (Ac3) does not inhibit HS biosynthesis, whereas 4F-GlcNAc (Ac3), 4-deoxy-GlcNAc (Ac3) (20), and 6F-GalNAc (Ac3) do. Also, although the effect of 4-deoxy-GlcNAc (Ac3) on CS/DS biosynthesis is similar to that of 4F-GlcNAc (Ac3), 4F-GalNAc (Ac3), and 6F-GalNAc (Ac3), the effect of 4-deoxy-GlcNAc (Ac3) on HS biosynthesis is much more pronounced (20). It is possible that these differences are due to disparities in the extent of reduction of UDP-GlcNAc levels, the inhibition of HS glycosyltransferases, or both. Furthermore, not all glycan structures are affected equally by the same sugar analog, possibly because of the different Km of the involved glycosyltransferases for their sugar nucleotides. For example, 4F-GlcNAc (Ac3) treatment reduces content and structural diversity of tri- and tetra-antennary, but not biantennary, N-linked glycans (28, 30). This is most likely because of higher Km values for UDP-GlcNAc of the N-acetylglucosaminyltranferases required for formation of tri- and tetra-antennary N-linked glycans, as indicated by Nishimura et al. (30). It is possible that a similar effect occurs with 4F-GalNAc (Ac3) and 6F-GalNAc (Ac3).
Figure 6.
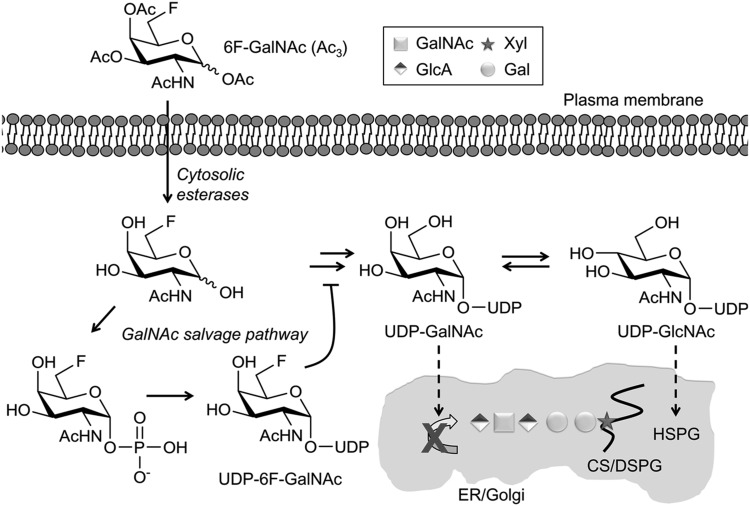
A sugar-nucleotide-mediated mechanism of inhibition of GAG biosynthesis by 6F-GalNAc (Ac3). Proposed mechanism: 6F-GalNAc (Ac3) enters the cell by passive diffusion, is deacetylated by aspecific cytosolic esterases, and becomes activated into UDP-6F-GalNAc by the machinery of the GalNAc salvage pathway. 6F-GalNAc is not incorporated into GAGs, but reduces the pool of natural UDP-GalNAc by using the same activation machinery or by a feedback mechanism. UDP-GlcNAc is also reduced, because UDP-GalNAc and UDP-GlcNAc are in equilibrium, catalyzed by an epimerase. It is likely that a part of UDP-6F-GalNAc is converted into UDP-6F-GlcNAc via the same epimerase reaction, thereby possibly contributing to the reduced level of UDP-GlcNAc via a nucleotide sugar feedback mechanism (18). As a result, the biosynthesis of GAGs and other glycans is reduced. The curving line represents a core protein. DSPG, chondroitin sulfate/dermatan sulfate proteoglycan. HSPG, heparan sulfate proteoglycan.
Of the 11 sugar analogs, 8 did not obviously affect biosynthesis of GAGs and other glycans, indicating that these analogs may not be able to enter the cell, are not transformed to their UDP-sugars, cannot access the endoplasmic reticulum/Golgi, or are not recognized by the relevant enzymes. No known pathway can transform 6-deoxy-GlcNAc and GlcA analogs into their UDP-sugars. In contrast to UDP activation of GalNAc, which can be directly phosphorylated at the C1 position, UDP activation of GlcNAc requires 2 phosphorylated intermediates: GlcNAc-6-PO4 and GlcNAc-1-PO4 (37). The lack of the 6-OH group excludes the possibility of formation of GlcNAc-6-PO4. Although some statistically significant decreases in GAG expression were observed for GlcA analogs (Supplemental Fig. S2B), it is unclear whether these changes represent a specific effect on GAG biosynthesis. Since UDP-GlcA is made of UDP-Glc, and GlcA is known to be directly activated only in plants (38), it is unlikely that UDP-2-deoxy-GlcA and UDP-4-deoxy-GlcA are formed. In addition, a concentration of 40 mM 4-deoxy-GlcA did not clearly reduce HS expression by SKOV3 cells (Supplemental Fig. S2B).
In conclusion, this study, together, with data in the literature, fuels the thought that a general mechanism underlies the observed effects of sugar analogs: the modified monosaccharides are taken up by cells and transformed to their activated glycosyl donors, thereby largely depleting naturally activated sugar pools, resulting in inhibition of glycan biosynthesis. Although not covered in our study, an additional inhibitory effect on glycosyltransferases is possible. Sugar analogs that do not affect glycan biosynthesis may not be transformed into their activated nucleotide sugars.
Supplementary Material
Acknowledgments
The authors thank G. ten Dam, J. Esko, B. Choudhury, F. Bonetto, A. Pistorius, A. Swolfs, R. Wismans, E. van de Westerlo, and T. van der Velden for their analytical and technical support. Lectins were generous gifts from the Departments of Nephrology/Cell Biology (Radboud University Medical Centre, Nijmegen, The Netherlands). This work was supported by the Dutch Cancer Society (Grant 2008-4058), the U.S. National Institutes of Health, National Institute of General Medical Sciences (Grant GM093131 to R.L.), and the Dutch Organization for Scientific Research (NWO Medium Investment Grant 40-00506-98-9001 and VIDI Grant 91713359 to D.J.L.). The authors declare no conflicts of interest.
Glossary
- AAL
Aleuria aurantia lectin
- AMAC
2-aminoacridone
- amu
atomic mass unit
- CAM
chicken chorioallantoic membrane
- ConA
concanavalin A
- CS
chondroitin sulfate
- DS
dermatan sulfate
- DSA
Datura stramonium
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- GAG
glycosaminoglycan
- GalNAc
N-acetyl-d-galactosamine
- GlcA
d-glucuronic acid
- GlcNAc
N-acetyl-d-glucosamine
- GNA
Galanthus nivalis agglutinin
- HexNAc
N-acetyl-hexosamine
- HPAEC
high-performance anion exchange chromatography
- HS
heparan sulfate
- HUVEC
human umbilical vein endothelial cell
- LCA
Lens culinaris agglutinin
- LC/MS
liquid chromatography/mass spectometry
- m/z
mass-to-charge ratio
- NRE
nonreducing end
- PNA
peanut agglutinin
- rh
recombinant human
- RP
reverse phase
- SNA
Sambucus nigra agglutinin
- UA
uronic acid
- UDP
uridine 5′-diphosphate
- VEGF
vascular endothelial growth factor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Bishop J. R., Schuksz M., Esko J. D. (2007) Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature 446, 1030–1037 [DOI] [PubMed] [Google Scholar]
- 2.Esko J. D., Selleck S. B. (2002) Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu. Rev. Biochem. 71, 435–471 [DOI] [PubMed] [Google Scholar]
- 3.Lindahl U., Li J. P. (2009) Interactions between heparan sulfate and proteins-design and functional implications. Int. Rev. Cell Mol. Biol. 276, 105–159 [DOI] [PubMed] [Google Scholar]
- 4.Theocharis A. D., Theocharis D. A. (2002) High-performance capillary electrophoretic analysis of hyaluronan and galactosaminoglycan-disaccharides in gastrointestinal carcinomas. differential disaccharide composition as a possible tool-indicator for malignancies. Biomed. Chromatogr. 16, 157–161 [DOI] [PubMed] [Google Scholar]
- 5.Pothacharoen P., Siriaunkgul S., Ong-Chai S., Supabandhu J., Kumja P., Wanaphirak C., Sugahara K., Hardingham T., Kongtawelert P. (2006) Raised serum chondroitin sulfate epitope level in ovarian epithelial cancer. J. Biochem. 140, 517–524 [DOI] [PubMed] [Google Scholar]
- 6.Ten Dam G. B., van de Westerlo E. M., Purushothaman A., Stan R. V., Bulten J., Sweep F. C., Massuger L. F., Sugahara K., van Kuppevelt T. H. (2007) Antibody GD3G7 selected against embryonic glycosaminoglycans defines chondroitin sulfate-E domains highly up-regulated in ovarian cancer and involved in vascular endothelial growth factor binding. Am. J. Pathol. 171, 1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreras C., Rushton G., Cole C. L., Babur M., Telfer B. A., van Kuppevelt T. H., Gardiner J. M., Williams K. J., Jayson G. C., Avizienyte E. (2012) Endothelial heparan sulfate 6-O-sulfation levels regulate angiogenic responses of endothelial cells to fibroblast growth factor 2 and vascular endothelial growth factor. J. Biol. Chem. 287, 36132–36146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Wijk X. M., van Kuppevelt T. H. (2014) Heparan sulfate in angiogenesis: a target for therapy. Angiogenesis 17, 443–462 [DOI] [PubMed] [Google Scholar]
- 9.Song K., Li Q., Peng Y. B., Li J., Ding K., Chen L., Shao C., Zhang L. J., Li P. (2011) Silencing of hHS6ST2 inhibits progression of pancreatic cancer through inhibition of Notch signaling. Biochem. J. 436, 271–282 [DOI] [PubMed] [Google Scholar]
- 10.Narita K., Staub J., Chien J., Meyer K., Bauer M., Friedl A., Ramakrishnan S., Shridhar V. (2006) HSulf-1 inhibits angiogenesis and tumorigenesis in vivo. Cancer Res. 66, 6025–6032 [DOI] [PubMed] [Google Scholar]
- 11.Berkin A., Szarek W. A., Kisilevsky R. (2000) Synthesis of 4-deoxy-4-fluoro analogues of 2-acetamido-2-deoxy-D-glucose and 2-acetamido-2-deoxy-D-galactose and their effects on cellular glycosaminoglycan biosynthesis. Carbohydr. Res. 326, 250–263 [DOI] [PubMed] [Google Scholar]
- 12.Dimitroff C. J., Kupper T. S., Sackstein R. (2003) Prevention of leukocyte migration to inflamed skin with a novel fluorosugar modifier of cutaneous lymphocyte-associated antigen. J. Clin. Invest. 112, 1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisilevsky R., Szarek W. A., Ancsin J. B., Elimova E., Marone S., Bhat S., Berkin A. (2004) Inhibition of amyloid A amyloidogenesis in vivo and in tissue culture by 4-deoxy analogues of peracetylated 2-acetamido-2-deoxy-alpha- and beta-d-glucose: implications for the treatment of various amyloidoses. Am. J. Pathol. 164, 2127–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laughlin S. T., Baskin J. M., Amacher S. L., Bertozzi C. R. (2008) In vivo imaging of membrane-associated glycans in developing zebrafish. Science 320, 664–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nigro J., Wang A., Mukhopadhyay D., Lauer M., Midura R. J., Sackstein R., Hascall V. C. (2009) Regulation of heparan sulfate and chondroitin sulfate glycosaminoglycan biosynthesis by 4-fluoro-glucosamine in murine airway smooth muscle cells. J. Biol. Chem. 284, 16832–16839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Wijk X. M., Oosterhof A., van den Broek S. A., Griffioen A. W., ten Dam G. B., Rutjes F. P., van Delft F. L., van Kuppevelt T. H. (2010) A 4-deoxy analogue of N-acetyl-D-glucosamine inhibits heparan sulphate expression and growth factor binding in vitro. Exp. Cell Res. 316, 2504–2512 [DOI] [PubMed] [Google Scholar]
- 17.Gloster T. M., Zandberg W. F., Heinonen J. E., Shen D. L., Deng L., Vocadlo D. J. (2011) Hijacking a biosynthetic pathway yields a glycosyltransferase inhibitor within cells. Nat. Chem. Biol. 7, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rillahan C. D., Antonopoulos A., Lefort C. T., Sonon R., Azadi P., Ley K., Dell A., Haslam S. M., Paulson J. C. (2012) Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pouilly S., Piller V., Piller F. (2012) Metabolic glycoengineering through the mammalian GalNAc salvage pathway. FEBS J. 279, 586–598 [DOI] [PubMed] [Google Scholar]
- 20.Van Wijk X. M., Thijssen V. L., Lawrence R., van den Broek S. A., Dona M., Naidu N., Oosterhof A., van de Westerlo E. M., Kusters L. J., Khaled Y., Jokela T. A., Nowak-Sliwinska P., Kremer H., Stringer S. E., Griffioen A. W., van Wijk E., van Delft F. L., van Kuppevelt T. H. (2013) Interfering with UDP-GlcNAc metabolism and heparan sulfate expression using a sugar analogue reduces angiogenesis. ACS Chem. Biol. 8, 2331–2338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beahm B. J., Dehnert K. W., Derr N. L., Kuhn J., Eberhart J. K., Spillmann D., Amacher S. L., Bertozzi C. R. (2014) A visualizable chain-terminating inhibitor of glycosaminoglycan biosynthesis in developing zebrafish. Angew. Chem. Int. Ed. Engl. 53, 3347–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ten Dam G. B., Yamada S., Kobayashi F., Purushothaman A., van de Westerlo E. M., Bulten J., Malmström A., Sugahara K., Massuger L. F., van Kuppevelt T. H. (2009) Dermatan sulfate domains defined by the novel antibody GD3A12, in normal tissues and ovarian adenocarcinomas. Histochem. Cell Biol. 132, 117–127 [DOI] [PubMed] [Google Scholar]
- 23.Van Wijk X. M., Vallen M. J., van de Westerlo E. M., Oosterhof A., Hao W., Versteeg E. M., Raben J., Wismans R. G., Smetsers T. F., Dijkman H. B., Schalkwijk J., van Kuppevelt T. H. (2012) Extraction and structural analysis of glycosaminoglycans from formalin-fixed, paraffin-embedded tissues. Glycobiology 22, 1666–1672 [DOI] [PubMed] [Google Scholar]
- 24.Ambrosius M., Kleesiek K., Götting C. (2008) Quantitative determination of the glycosaminoglycan delta-disaccharide composition of serum, platelets and granulocytes by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1201, 54–60 [DOI] [PubMed] [Google Scholar]
- 25.Lawrence R., Olson S. K., Steele R. E., Wang L., Warrior R., Cummings R. D., Esko J. D. (2008) Evolutionary differences in glycosaminoglycan fine structure detected by quantitative glycan reductive isotope labeling. J. Biol. Chem. 283, 33674–33684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hart G. W., Akimoto Y. (2009) The O-GlcNAc Modification. In Essentials of Glycobiology Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds, p. 263–279, Cold Spring Harbor Laboratory Press, New York, USA: [PubMed] [Google Scholar]
- 27.Dimitroff C. J., Bernacki R. J., Sackstein R. (2003) Glycosylation-dependent inhibition of cutaneous lymphocyte-associated antigen expression: implications in modulating lymphocyte migration to skin. Blood 101, 602–610 [DOI] [PubMed] [Google Scholar]
- 28.Barthel S. R., Antonopoulos A., Cedeno-Laurent F., Schaffer L., Hernandez G., Patil S. A., North S. J., Dell A., Matta K. L., Neelamegham S., Haslam S. M., Dimitroff C. J. (2011) Peracetylated 4-fluoro-glucosamine reduces the content and repertoire of N- and O-glycans without direct incorporation. J. Biol. Chem. 286, 21717–21731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marathe D. D., Buffone A. Jr., Chandrasekaran E. V., Xue J., Locke R. D., Nasirikenari M., Lau J. T., Matta K. L., Neelamegham S. (2010) Fluorinated per-acetylated GalNAc metabolically alters glycan structures on leukocyte PSGL-1 and reduces cell binding to selectins. Blood 115, 1303–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura S., Hato M., Hyugaji S., Feng F., Amano M. (2012) Glycomics for drug discovery: metabolic perturbation in androgen-independent prostate cancer cells induced by unnatural hexosamine mimics. Angew. Chem. Int. Ed. Engl. 51, 3386–3390 [DOI] [PubMed] [Google Scholar]
- 31.Sorrell J. M., Carrino D. A., Caplan A. I. (1993) Structural domains in chondroitin sulfate identified by anti-chondroitin sulfate monoclonal antibodies: immunosequencing of chondroitin sulfates. Matrix 13, 351–361 [DOI] [PubMed] [Google Scholar]
- 32.Ly M., Leach F. E. III, Laremore T. N., Toida T., Amster I. J., Linhardt R. J. (2011) The proteoglycan bikunin has a defined sequence. Nat. Chem. Biol. 7, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Wijk X. M., Oosterhof A., Versteeg E. M., van de Westerlo E. M., van Kuppevelt T. H. (2014) ‘Immunosequencing’ of heparan sulfate from human cell lines and rat kidney: the (GlcNS6S-IdoA2S)₃ motif, recognized by antibody NS4F5, is located towards the non-reducing end. Biochem. J. 461, 461–468 [DOI] [PubMed] [Google Scholar]
- 34.Bernacki R. J., Sharma M., Porter N. K., Rustum Y., Paul B., Korythyk W. (1977) Biochemical characteristics, metabolism, and antitumor activity of several acetylated hexosamines. J. Supramol. Struct. 7, 235–250 [DOI] [PubMed] [Google Scholar]
- 35.Pouilly S., Bourgeaux V., Piller F., Piller V. (2012) Evaluation of analogues of GalNAc as substrates for enzymes of the mammalian GalNAc salvage pathway. ACS Chem. Biol. 7, 753–760 [DOI] [PubMed] [Google Scholar]
- 36.Zandberg W. F., Kumarasamy J., Pinto B. M., Vocadlo D. J. (2012) Metabolic inhibition of sialyl-Lewis X biosynthesis by 5-thiofucose remodels the cell surface and impairs selectin-mediated cell adhesion. J. Biol. Chem. 287, 40021–40030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freeze H. H., Elbein A. D. (2009) Glycosylation precursors. In Essentials of Glycobiology Varki A., Cummings R. D., Esko J. D., Freeze H. H., Stanley P., Bertozzi C. R., Hart G. W., Etzler M. E., eds, p. 47–61, Cold Spring Harbor Laboratory Press, New York, USA: [PubMed] [Google Scholar]
- 38.Bar-Peled M., O’Neill M. A. (2011) Plant nucleotide sugar formation, interconversion, and salvage by sugar recycling. Annu. Rev. Plant Biol. 62, 127–155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.