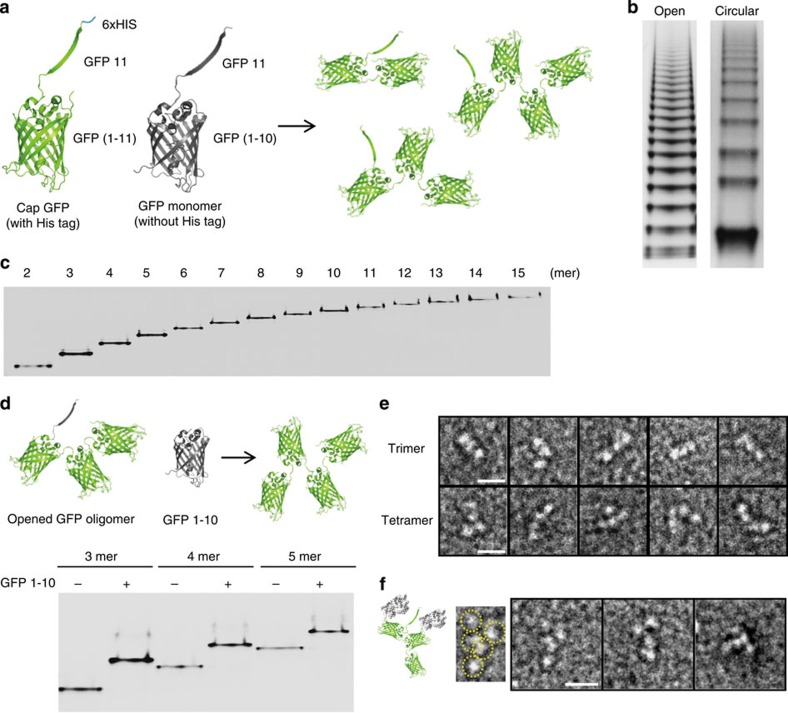
Figure 4. Fabrication and characterization of linearly opened GFP oligomers.
(a) Schematic representation of the construction of linearly opened GFP oligomers. CapGFP is designed to contain the GFP 11 strand connected to the N terminus of full-length GFP (1–11) with a His tag. Both CapGFP and GFP monomer (without His tag) were co-expressed in cells and opened oligomers were purified by His-affinity purifications. (b) Native PAGE analysis of open and circular forms of GFP oligomers. (c) Analysis of discrete opened oligomers from dimer to pentadecamer by native PAGE. (d) In-vitro assemblies of opened GFP oligomers with the GFP 1–10 fragment. Linearly opened GFP trimer, tetramer and pentamer were reacted with excess GFP 1–10 and resulting protein assemblies were analysed in a native PAGE gel. (e) TEM images of opened GFP trimer and tetramer. (f) TEM images and schematic drawing of MBP-displayed opened GFP trimer. A possible protein arrangement in a representative TEM image (a copy of the first image) was suggested with dotted yellow circles. Scale bars, 10 nm.