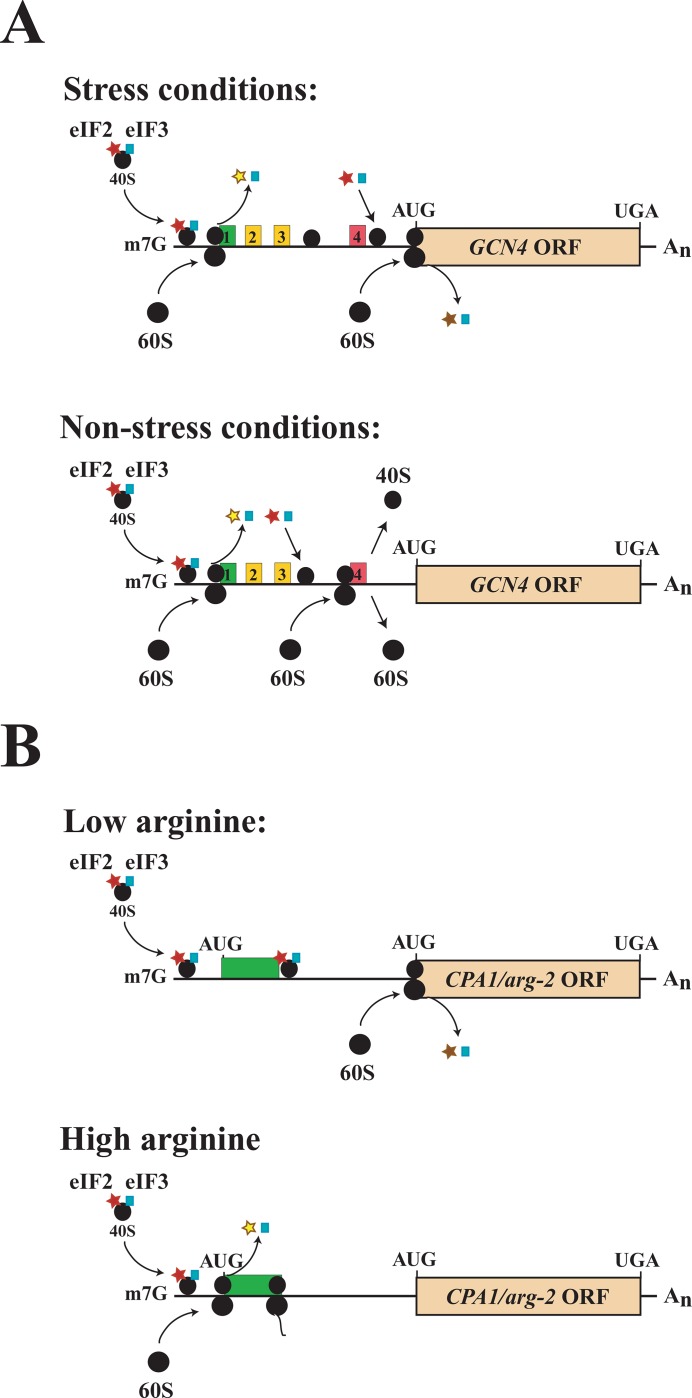
Figure 5. Upstream open reading frame-mediated regulation of translation.
(A) The upstream open reading frames (uORFs) in yeast GCN4 mRNA regulate translation of the GCN4 ORF through a re-initiation mechanism controlled by amino acid biosynthesis availability. The GCN4 uORFs are no more than a few codons long and their encoded proteins have no function. Under non-starvation conditions, translation initiates at uORF1, but, upon termination, up to 50% of the 40S ribosomal subunits remain associated with the mRNA. Retention of the 40S subunits is due to sequences immediately upstream and downstream of the uORF1 start and stop codons, respectively, which interact with the translation initiation factor, eIF3a, which normally is released from the 40S subunit after initiation. Retention of eIF3 enables the 40S subunit to resume scanning and re-initiate translation. The 40S subunit regains competency to recognize initiation codons quickly because of the binding of a new eIF2-tRNAi-GTP ternary complex. Translation of uORF4 leads to full ribosome dissociation at its termination codon, preventing re-initiation at the GCN4 ORF [85]. Amino acid starvation results in reduced levels of ternary complex through the GCN2-mediated phosphorylation of the eIF2α subunit, which inhibits its recycling into a new ternary complex. As a result of the reduced availability of ternary complex, a 40S subunit that has resumed scanning after uORF1 translation will scan past uORF4 before a ternary complex binds, thus enabling the 40S subunit to reach the GCN4 ORF to initiate protein synthesis. (B) The single uORF in yeast CPA1/Neurospora crassa arg-2 mRNAs regulates translation through a ribosome-stalling mechanism controlled by arginine availability. Translation of the uORF-encoded, nascent arginine attenuator peptide (AAP) in response to arginine causes ribosome stalling at the uORF termination codon. This in turn inhibits leaky scanning of any 40S ribosomal subunits through the uORF and thus prevents them from reaching the main ORF. The amino acid sequence of the AAP peptide is key to the negative cis-acting regulation, which in the presence of arginine may interact with the ribosome tunnel near the large ribosomal protein L22 to cause stalling [88]. For peptide-mediated regulators, the uORFs that encode them are typically longer, as the regulation is mediated by the encoded protein. Translation of uORFs may also trigger nonsense-mediated decay (NMD) of the mRNA in addition to the translational regulation, thus serving as another means to regulate expression. For example, translation of the CPA1 uORF induces NMD whereas translation of the GCN4 ORFs does not [88].