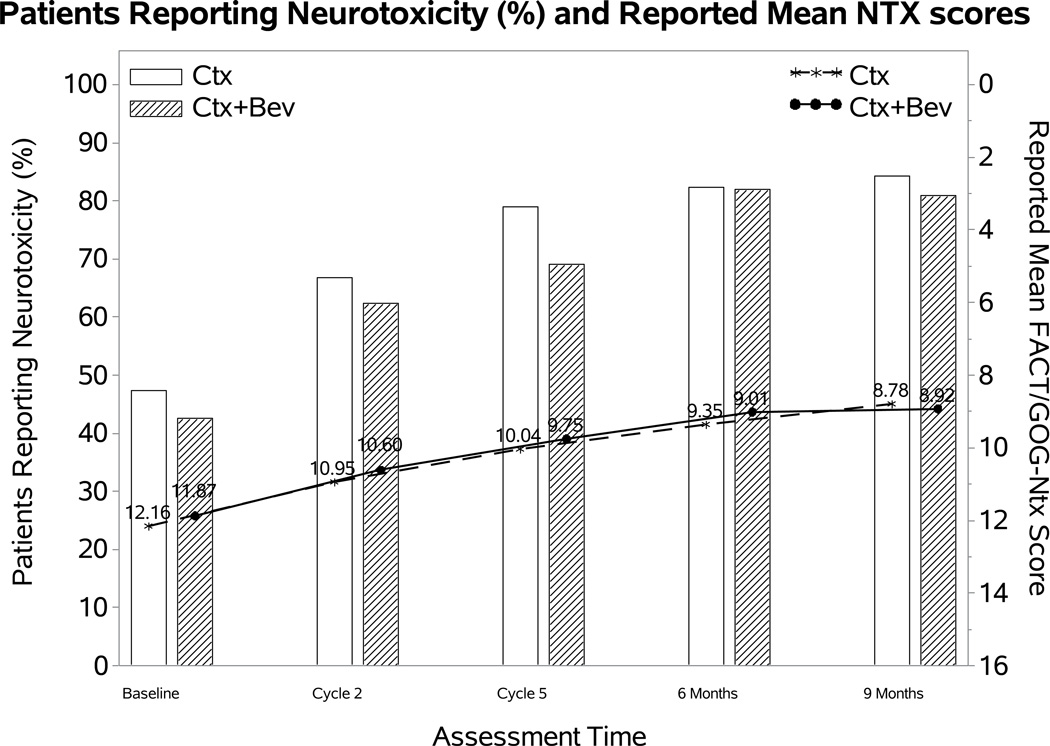
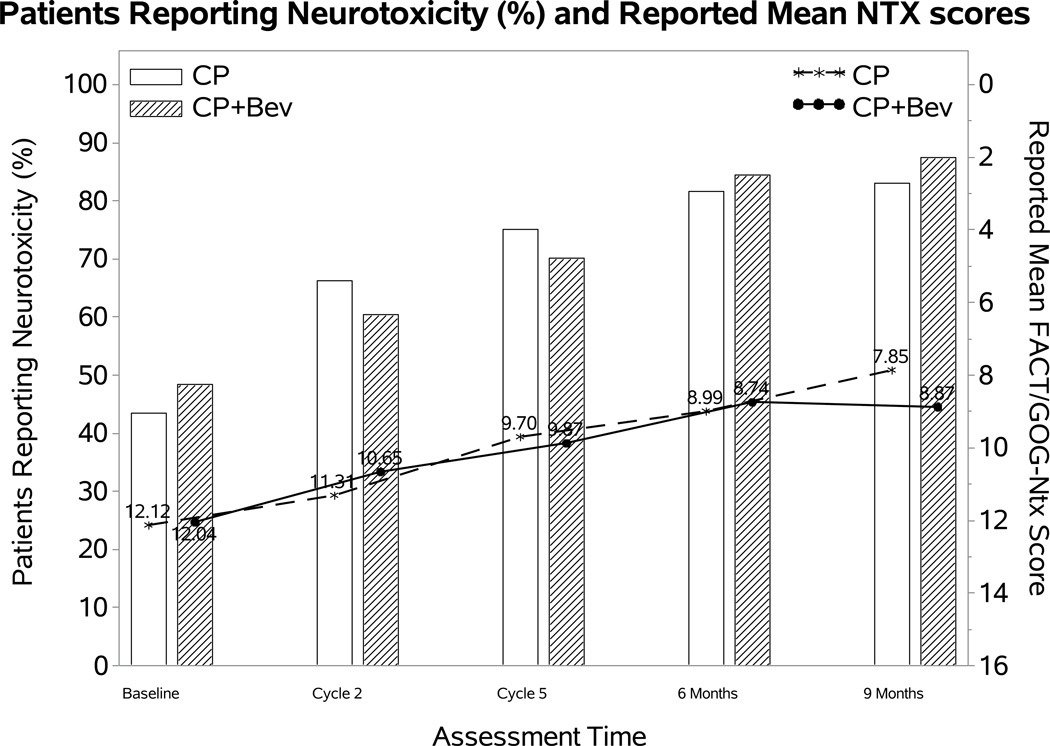
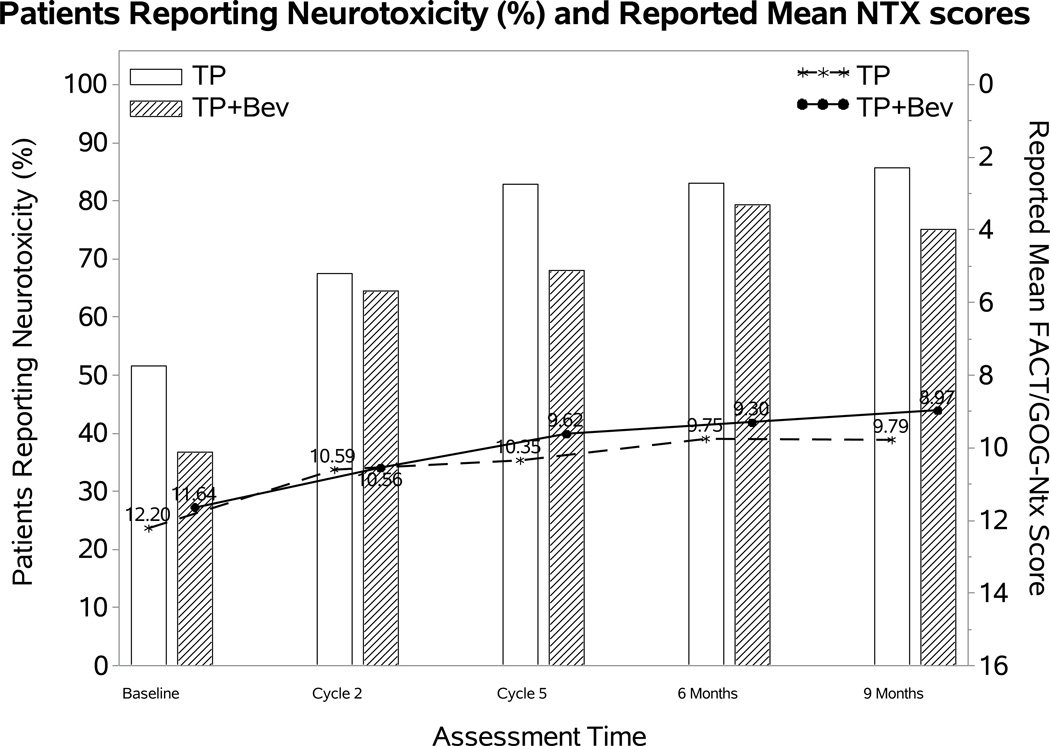
Figure 2. Patient-reported outcomes (PROs) using the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group (FACT/GOG) Neurotoxicity subscale (Ntx).
During the period of assessment, patients treated with chemotherapy plus bevacizumab as well as those on the topotecan-paclitaxel backbone who received bevacizumab had less odds of reporting neurotoxicity. Among those patients reporting neurotoxcity there were no significant differences in severity of neurotoxic symptoms for the following treatment groups: chemotherapy alone (both backbones) vs chemotherapy (both backbones) with bevacizumab (Panel 2A); cisplatin and paclitaxel with and without bevacizumab (Panel 2B); topotecan and paclitaxel with and without bevacizumab (Panel 2C). Ctx: chemotherapy; Bev: bevacizumab; CP: cisplatin and paclitaxel; TP: topotecan and paclitaxel.