Figure 9.
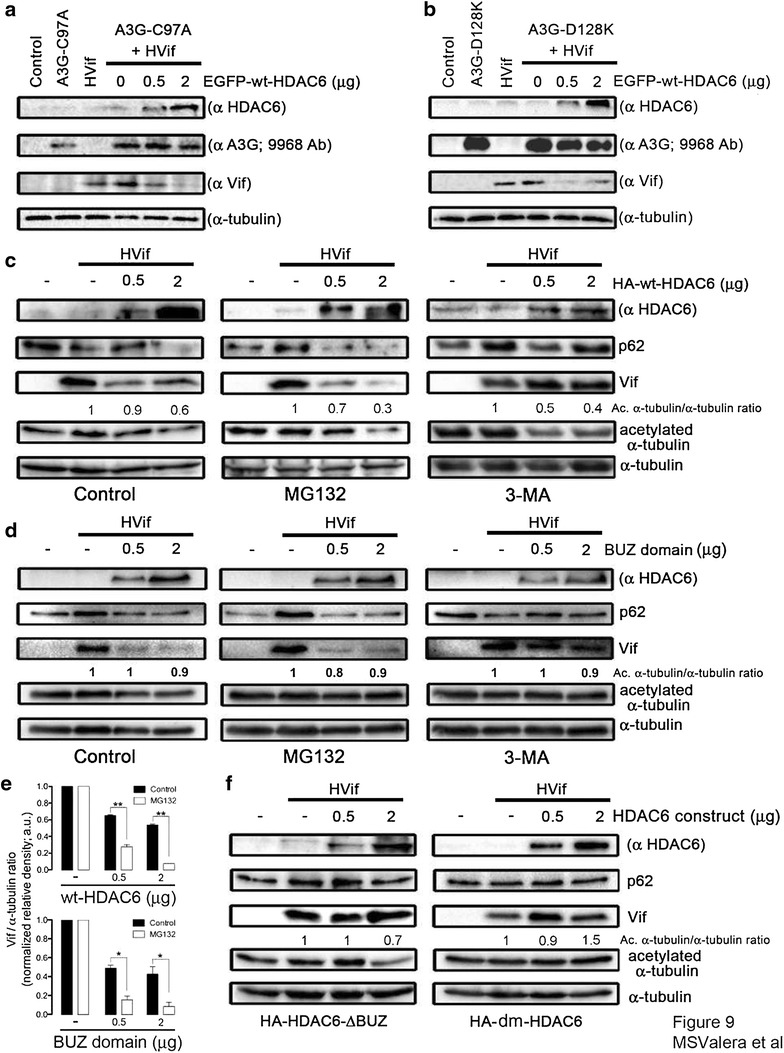
The autophagic degradation of Vif is mediated by the BUZ domain and deacetylase activity of HDAC6. a, b Western blot of HDAC6-mediated Vif degradation in HEK 293T cells co-expressing the A3G-C97A (a) or A3G-D128K (b) mutants resistant to Vif. c, d Western blot of HDAC6- or BUZ-mediated Vif degradation concomitant with p62 analysis in HEK 293T cells co-expressing Vif and HA-wt-HDAC6 or HA-BUZ in the absence of A3G, or in cells treated with MG132 or 3-MA inhibitors. e Histograms show quantification of the positive effect exerted by proteasomal inhibition by MG132 on wt-HDAC6- and BUZ-mediated Vif autophagic degradation. Data are from three independent western-blot of wt-HDAC6 and BUZ domain constructs, as presented in (c–d), comparing Vif/α-tubulin ratios in Control and MG132 experimental conditions. Data are mean ± S.E.M. of three independent experiments carried out in triplicate: ** and * are p < 0.01 and p < 0.05, respectively, Student’s t test. f Western blot of HA-HDAC6-ΔBUZ- and HA-dm-HDAC6-effect on Vif and p62 stability in HEK 293T cells in the absence of A3G. From a to f, when indicated, control untreated cells, cells only expressing Vif or each A3G construct or endogenous HDAC6 are shown. When indicated, HDAC6 constructs-deacetylase activity against acetylated α-tubulin is shown. α-tubulin is the control for total protein. Data are representative of three independent experiments.
