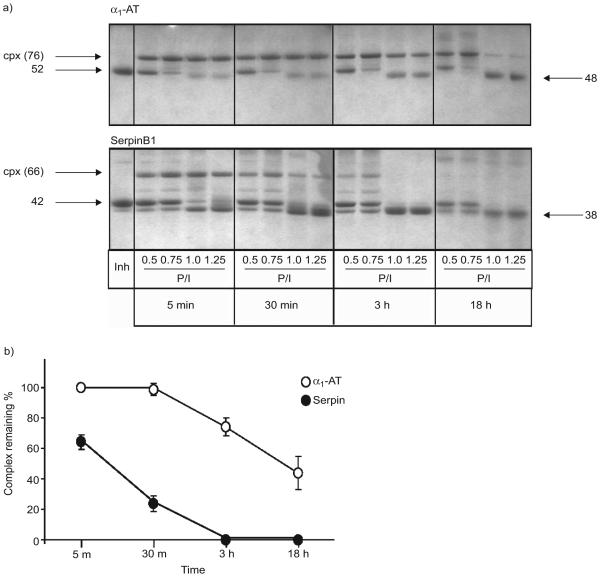
FIGURE 5.
a) Reaction of α1-antitrypsin (α1-AT) and SerpinB1 with elastase and stability over time of the resulting covalent complexes. α1 AT or SerpinB1 (1.3 mM) were allowed to react with varying molar ratios of elastase (indicated by protease:inhibitor (P/I) ratio ranging from subthreshold to excess elastase. The reactions were stopped after 5 min, 30 min, 3 h or 18 h, and the products analysed on Coomassie blue stained Bis Tris gels. α1-AT (top panel, left lane) migrates at 52 kDa and its complex (cpx) with elastase at 76 kDa; pure SerpinB1 (bottom panel, left lane) migrates at 42 kDa and its cpx with elastase at 66 kDa. b) Quantitation of α1-AT–elastase and SerpinB1–elastase complexes remaining over time for the reactions with excess elastase (P/I 1.25). The 5-min values were considered 100%. Data are presented as mean±SEM of three experiments.