Abstract
In the last years there is an increasing trend towards personalized medicine for patients with asthma. This is due to the availability of novel specific therapies. These new compounds are supposed to be used in well-defined patient groups, which are likely to respond to these interventions. In addition to already used anti-IgE, novel monoclonal antibodies such as anti-IL-5 and anti-IL-13 are becoming available. Currently clinical trials are ongoing to identify which patient population will respond to these novel therapies.
Key words: Asthma, Inflammation, Phenotypes, Monoclonal antibodies
Introduction
In recent years, many areas of medicine have seen an ever increasing use of personalized therapy options for specific disease phenotypes. An example in pneumology particularly worthy of note is the targeted therapy used in patients with non-small cell lung cancer and confirmed mutations in certain growth factor receptors. Specific therapies have also been developed for cystic fibrosis patients with particular mutations. The most important advance in this context, however, lies in the strategy whereby these new therapies are used only in those patients identified prior to treatment (by determining and analyzing certain parameters, e. g., mutations in growth receptors) as having a high likelihood of benefitting from a targeted therapy, rather than using treatments in an untargeted manner in all patients with a particular disorder. A similar development can also be observed in the treatment of asthma patients. Our pathophysiological understanding of this disease has altered significantly in recent years. It is now well established that the large group of people with an asthma diagnosis is in fact a highly heterogenous group exhibiting varying degrees of disease severity. Further developments have been made in recent years in the classification of patients into different phenotypes and endotypes [1]. Division into phenotypes is based on the use of various clinical or immunological characteristics which subdivide patients into different subgroups. A simple yet relevant example of this is the subdivision into allergic and non-allergic asthma. Further classification is possible on the basis of the inflammatory reaction detectable in the airways. In this context, patients exhibiting an eosinophilic inflammatory response in the airways (eosinophilic asthma) represent an important group of patients compared with patients in whom no signs of eosinophilic inflammation can be detected [2]. Another recent development has been the description of endotypes [3]. The concept of endotypes involves an understanding of the pathophysiological causes of a disease and applying this understanding in the use of specific therapies. This concept is far from fully elaborated and, to date, only a small number of endotypes have been described in detail. Patients with a T-helper cell 2 (Th2)-induced inflammatory response represent one of these endotypes.
Different inflammatory phenotypes
It has long been know that an inflammatory response can be detected in the airways of bronchial asthma patients. An increased eosinophil, mast cell, as well as B and Th2 cell count was initially considered characteristic of the inflammatory response seen in these patients [4]. Th2 cells are CD4-positive T cells that produce certain marker cytokines, including interleukin (IL)-4, IL-5, and IL-13 [5]. However, it has since become evident that other inflammatory patterns can also be detected in asthma patients (Fig. 1). With the establishment of sputum diagnosis as a non-invasive procedure, it became possible to collect data on the inflammatory response in asthma patients in clinical studies. However, measuring eosinophils in sputum is time-consuming and not feasible in daily clinical routine. Therefore, the blood eosinophil count — an approach that already had its supporters 40 years ago — represents a further parameter for describing eosinophilic inflammation [6]. A normal blood eosinophil count in healthy adults is between 15 and 650 cells/ l, with considerable circadian variation (low values in the morning, high at night) [7]. Recent studies classified eosinophil counts in asthma patients into threecategories: < 300 cells/μl, normal; 300–500 cells/μl, moderately elevated; and > 500 cells/μl, high [8].
Fig. 1:
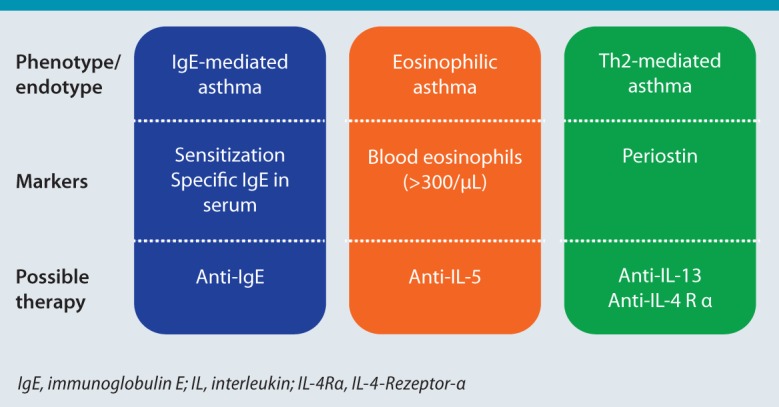
The concept of different asthma phenotypes and possible specific treatments.
Other inflammatory cells, e. g., neutrophils, are detected in the airways of some patients [9]. Other inflammatory phenotypes include patients with mixed eosinophilic/neutrophilic inflammation or patients with no significant inflammatory response. Recent large-scale studies have shown that an eosinophilic inflammatory response is detectable in approximately 50 % of patients. Interestingly, however, the inflammatory phenotype was not stable in all patients, but subject instead to alteration over time.
Drug treatment of asthma patients is based on the administration of inhaled steroids, possibly in combination with inhaled bronchodilators [10], and this concept has changed little in recent years. However, it is not effective in controlling symptoms in all patients and a further therapy escalation is recommended in those not responding sufficiently in order to achieve better disease control. This applies in particular to patients with severe asthma already using high-dose inhaled steroids and bronchodilators. Disease in this patient group is often inadequately controlled [11]; however, further treatment options remain limited here. Alongside the use of systemic steroids — with their known side effects — treatment with anti-immunoglobulin E (IgE) is approved only for patients with severe allergic bronchial asthma. According to the new recommendations of the American Thoracic Society (ATS) and European Respiratory Society (ERS) on the diagnosis and treatment of severe allergic bronchial asthma, anti-IgE therapy is an intervention specifically recommended for patients with severe allergic asthma [12].
The IgE-mediated asthma phenotype
In IgE-mediated asthma, allergen exposure causes increased inflammation and a deterioration in lung function. Varying degrees of disease severity are similarly observed in this patient group. A new and specific treatment approach has also been approved for patients with severe allergic asthma since 2005. The IgE-specific monoclonal antibody omalizumab can be used in these patients as an add-on treatment. Following subcutaneous injection, omalizumab binds to free-circulating IgE and prevents it from binding to IgE receptors on mast cells and basophils. Clinical studies show a reduction in the exacerbation rate of up to 50 %, significantly fewer cases of emergency treatment, and often also an improvement in lung function [13]. Under omalizumab therapy, oral steroids can frequently be discontinued and the dose of inhaled steroids reduced [14]. Omalizumab treatment in severe allergic asthma is initially administered over a 4-month period, followed by an evaluation of therapy response according to clinical criteria (lung function, degree of asthma control, exacerbation, etc.). Treating the clinical phenotype of severe allergic asthma with omalizumab is a good example of the therapeutic and prognostic relevance of asthma phenotyping [1, 15].
Two recent studies re-investigated the efficacy of omalizumab in children and adults. In one study, 419 urban children and young adults with persistent asthma were treated with either omalizumab or placebo, alongside standard therapy, for 60 weeks [16]. One important finding of this study was an almost 25 % reduction in the number of days on which asthma symptoms were experienced over a 2-week period. Furthermore, a reduction in the number of subjects with exacerbations was observed. Omalizumab therapy also resulted in a reduction in ICS and long-acting β agonist (LABA) dose. The effect of Omalizumab on the number of exacerbations was present to the same degree in both spring and autumn, although no reduction in the number of viral infections was observed. Even in patients with uncontrolled asthma despite high-dose inhaled therapy (ICS and LABA), omalizumab therapy was able to achieve a relative reduction in exacerbations of 25 %. Furthermore, omalizumab treatment resulted in improved symptom scores, a reduction in pro re nata medication, and a decrease in symptoms compared with placebo [17]. The current ERS and ATS guidelines on the treatment of severe asthma also recommend a 4-month trial treatment for patients with severe allergic asthma and treatment continuation if effective [12]. Treatment duration has hitherto been a subject of discussion. There have been no reliable data to date on how long treatment should be continued. Whilst model calculations suggest that a significant reduction in IgE production can possibly be expected after five years, the clinical effect of this IgE reduction has not yet been investigated, and no recommendation on stopping or interrupting treatment can be made [18].
A further interesting development is that anti-IgE treatment is apparently also effective in severe asthma patients in whom no sensitization to aeroallergens can be detected using conventional methods. A small initial study showed an improvement in lung function and a trend towards a reduction in acute exacerbations [19]. The efficacy of anti-IgE appears not to be based on the reduction in free IgE and regulation of high-affinity IgE receptors alone. Direct effects on certain populations of dendritic cells, which play a particularly important role in the defense against viral infections, have also been described. This could explain the efficacy of anti-IgE in patients with “non-allergic” asthma [20].
Patients with asthma and eosinophilic inflammation
Patients with severe asthma and eosinophilic inflammation represent another interesting group. These patients may experience recurrent exacerbations associated with inflammation of the airways. The cytokine IL-5 is an important mediator of eosinophils; it is essential not only for the migration of eosinophils to the lung, but also for the lifespan of these cells. Experience gained in patients with hypereosinophilic syndrome (HES) has shown that administering monoclonal antibodies against IL-5 can dramatically reduce eosinophil count, permitting in turn a reduction in systemic steroid therapy [21]. In addition, ever more data to support the efficacy of anti-IL-5 treatment in patients with asthma and eosinophilic inflammation are becoming available [22, 23]. A large phase-II study investigated the response to monoclonal anti-IL-5 antibodies in asthma patients with signs of eosinophilic inflammation (percentage of eosinophils in sputum ≥ 3 % or blood eosinophil count ≥ 300/μl) and a history of recurrent asthma exacerbations [24]. The main effect of this therapy was a reduction in exacerbations, an effect observed even at the lowest dose of monoclonal antibodies. Studies with another monoclonal antibody against IL-5 showed beneficial effects also on lung function parameters in patients with eosinophilic inflammation [25]. Despite the positive effects of anti-IL-5 treatment observed to date in this patient group, evidence of the efficacy of these new approaches in large phase-III studies is still lacking. An important parameter in the identification of patients who may respond to this type of therapy appears to be the testing and demonstration of eosinophilic inflammation. In this context, detection of these cells in the lung would theoretically be optimal. However, sputum and bronchoalveolar lavage are both time-consuming procedures that are not suited to standard use in the routine diagnosis of this patient group. For this reason, focus has recently shifted more in the direction of evaluating peripheral blood parameters as possible biomarkers. One interesting parameter appears to be the eosinophil count in peripheral blood as a method of detecting an eosinophilic inflammatory response. It is important here to measure the absolute (not the relative) number of blood eosinophils. Patients in whom an eosinophil count exceeding 300/μl was detected were included in clinical studies [24]. However, further investigations are required to define appropriate threshold values in order to identify potential responder populations more accurately. It should also be noted that antibodies binding to the IL-5 receptor are also undergoing clinical trials. These antibodies neutralize the cytokine IL-5 via direct blockade of the receptor on the cells [26]. It remains to be seen whether this approach differs in terms of clinical efficacy from direct IL-5 inhibition. However, it is important to bear in mind that approximately 50 % of asthma exacerbations are not characterized by airway inflammation. At present, there are no promising therapy options for this patient population [27].
Patients with Th2-induced inflammation
As discussed above, the Th2-induced inflammatory response plays an important role particularly in allergic asthma. Besides IL-5, notably IL-4 and IL-3 are considered key cytokines here [5]. Specific antibodies that either neutralize these cytokines or block the relevant receptor have now been developed. Precise phenotyping of asthma patients also depends on the development of biomarkers that may help in the selection of suitable patients. One study of particular interest in this regard was able to identify asthma patients who would respond particularly well to treatment with a monoclonal antibody to IL-13 by measuring serum periostin [28]. This study shows that the description of relevant phenotypes of asthma and the effective treatment of these patients with specific drug therapy is becoming increasingly successful. Interestingly, IL-3 inhibition has been observed to improve lung function and reduce exhaled NO. This improvement appears to be independent of the eosinophilic inflammatory response in the airways, which is little affected by treatment. This means that treatment is effective despite detectable persisting eosinophilic inflammation. Animal models have already shown that the principal effect of IL-13-targeted treatment is reduced hyperresponsiveness and mucus production in the airways — with little effect on the inflammatory response [29]. IL-13-induced cellular activation requires binding to the IL-4 receptor α (IL-4Rα) chain, which, together with the IL-13 receptor-1 chain, forms an important IL-13 receptor [30]. Interestingly, the IL-4Rα chain is also an essential component of the IL-4 receptor. This means that blockade of the IL-4Rα chain inhibits not only IL-4 action, but also IL-13 action (Fig. 2). A corresponding monoclonal antibody is already in clinical testing. One study on patients with asthma and eosinophilic inflammation demonstrated that antibody treatment resulted in improved lung function as well as reduced exhaled NO and exacerbations (despite a simultaneous reduction in conventional treatment) [31]. Further studies are required to establish the clinical relevance of these promising effects. A very recent study shows that these antibodies can also significantly improve disease activity in atopic dermatitis patients [32]. In patients with the combination of severe asthma and atopic dermatitis, these new antibodies could make it possible to treat both morbidities.
Fig. 2:
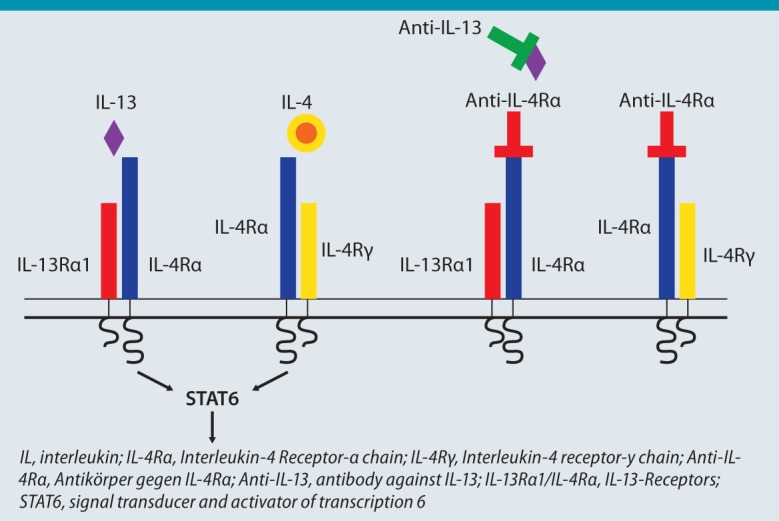
Structure of the interleukin-4 receptor comprising the IL-4 receptor-α chain, the γ chain, and the IL-13 receptor. IL-13 can be directly blocked by an antibody. An antibody against IL-4Rα blocks binding of both IL-4 and IL-13 to the receptor.
It has become evident in recent years that the airway epithelium also plays an important role in the initiation of an allergic airway inflammatory response. Important mediators in this context include IL-25, IL-33, and thymic stromal lymphopoietin (TSLP). TSLP interacts with a variety of immune cells and experimental models have shown the crucial role of TSLP in the initiation of a Th2 response. A recent study investigated a newly developed monoclonal antibody to TSLP in patients with allergic asthma. Antibody treatment resulted in a reduction in early and late allergic reactions following inhaled allergen challenge in patients with mild allergic asthma [33]. Data is as yet insufficient to answer the question of whether this approach is effective in the patient group with severe asthma or in particular phenotypes.
The interaction between OX40 and OX40 ligand (OX40L) is another novel approach investigated to date only in animal studies. Expression of the costimulatory molecule OX on T cells and OX40L on antigen-presenting cells plays an important role in maintaining and reactivating T-effector memory cells. In clinical studies, however, the use of a monoclonal antibody to OX40L had no effect on early and late allergy-induced reactions in patients with mild allergic asthma [33].
Non-eosinophilic asthma
One group of patients for whom no advances in treatment have been seen is patients with disease that shows no evidence of eosinophilic inflammation. These patients may experience strong symptoms despite the absence of any significant detectable eosinophilic inflammation [34]. Patients are often female and overweight and it is important, particularly in this group, that an asthma diagnosis be confirmed by an experienced physician. In clinical studies, treatment with a macrolide antibiotic agent (azithromycin) led to a reduction in acute exacerbations [35]. However, these results were observed in a subgroup analysis of a large trial, meaning that no recommendation for the regular use of azithromycin in patients with severe asthma can be made at present [12]. IL-17A is considered an interesting cytokine in the development of neutrophilic inflammation. Here too, monoclonal antibodies that neutralize this cytokine through blockade of the relevant receptor are being developed. A study published recently also showed that the use of this antibody to treat asthma patients is safe. However, there is no evidence as yet that this antibody has an effect on lung function or symptoms in asthma patients [36].
Summary
Recent advances in the treatment of patients with severe asthma are moving increasingly towards the use of specific therapies, in particular monoclonal antibodies. In addition to the anti-IgE already available, anti-IL-5, anti-IL-13, and anti-IL-4Rα have all been tested in clinical trials with good results in some cases. All these approaches are particularly effective in patients with eosinophilic inflammation (both with and without detectable allergy). Thus, it remains to be seen how these novel therapeutic options — should they be approved for treatment — will be positioned in the treatment guidelines compared with those available to date. What is certain is that a good clinical characterization combined with additional patient markers is required. Besides blood eosinophils (absolute cell count), it remains to be seen whether new tests like serum periostin have a contribution to make. One group of patients for whom no new developments are in sight is the group with non-eosinophilic inflammation. It is precisely here that further research needs to be undertaken to establish novel treatment options.
Abbreviations
- ATS
American Thoracic Society
- ERS
European Respiratory Society
- HES
Hypereosinophilic syndrome
- ICS
Inhaled corticosteroids
- IgE
Immunoglobulin E
- IL
Interleukin
- IL-4Rα
IL-4 receptor α
- LABA
Long-acting β agonists
- NO
Nitric oxide
- OX40L
OX40 ligand
- Th
Thelper cells
- TSLP
Thymic stromal lymphopoietin
Footnotes
Conflict of interest
The author has received fees from Boehringer Ingelheim, Novartis, Almirall, AstraZeneca, Chiesi und GlaxoSmith-Kline for oral presentations and/or advisory boards.
Cite this as
Taube C. Bronchial Asthma: is personalized therapy on the horizon? Allergo J Int 2014;23:246–51 DOI: 10.1007/s40629-014-0028-y
References
- 1.Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42:650–8. doi: 10.1111/j.1365-2222.2011.03929.x. [DOI] [PubMed] [Google Scholar]
- 2.Taube C, Buhl R. Does phenotyping asthma help to improve differential treatment? Dtsch Med Wochenschr. 2010;135:468–73. doi: 10.1055/s-0030-1249191. [DOI] [PubMed] [Google Scholar]
- 3.Lotvall J, Akdis CA, Bacharier LB, Bjermer L, Casale TB, Custovic A, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127:355–60. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 4.Holgate ST. Innate and adaptive immune responses in asthma. Nat Med. 2012;18:673–83. doi: 10.1038/nm.2731. [DOI] [PubMed] [Google Scholar]
- 5.Taube C, Dakhama A, Gelfand EW. Insights into the pathogenesis of asthma utilizing murine models. Int Arch Allergy Immunol. 2004;135:173–86. doi: 10.1159/000080899. [DOI] [PubMed] [Google Scholar]
- 6.Horn BR, Robin ED, Theodore J, Van KA. Total eosinophil counts in the management of bronchial asthma. N Engl J Med. 1975;292:1152–5. doi: 10.1056/NEJM197505292922204. [DOI] [PubMed] [Google Scholar]
- 7.Winkel P, Statland BE, Saunders AM, Osborn H, Kupperman H. Within-day physiologic variation of leukocyte types in healthy subjects as assayed by two automated leukocyte differential analyzers. Am J Clin Pathol. 1981;75:693–700. doi: 10.1093/ajcp/75.5.693. [DOI] [PubMed] [Google Scholar]
- 8.Malinovschi A, Fonseca JA, Jacinto T, Alving K, Janson C. Exhaled nitric oxide levels and blood eosinophil counts independently associate with wheeze and asthma events in National Health and Nutrition Examination Survey subjects. J Allergy Clin Immunol. 2013;132:821–7. doi: 10.1016/j.jaci.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype. J Allergy Clin Immunol. 2007;119:1043–52. doi: 10.1016/j.jaci.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 10.Korn S, Taube C, Buhl R. Treatment strategies for asthma. Internist. 2012;53:429–38. doi: 10.1007/s00108-011-3001-6. [DOI] [PubMed] [Google Scholar]
- 11.Korn S, Both J, Jung M, Hubner M, Taube C, Buhl R. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474–9. doi: 10.1016/j.anai.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–73. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 13.Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–31. doi: 10.1016/j.rmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Braunstahl GJ, Chlumsky J, Peachey G, Chen CW. Reduction in oral corticosteroid use in patients receiving omalizumab for allergic asthma in the real-world setting. Allergy Asthma Clin Immunol. 2013;9:47. doi: 10.1186/1710-1492-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64:1728–36. doi: 10.1111/j.1398-9995.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 16.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011;154:573–82 [DOI] [PubMed]
- 18.Schreiber J, Kopp MV, Korn S, Taube C, Buhl R. Disease modification and duration of omalizumab treatment in patients with severe allergic asthma. Pneumologie. 2014;68:187–92. doi: 10.1055/s-0033-1359242. [DOI] [PubMed] [Google Scholar]
- 19.Garcia G, Magnan A, Chiron R, Contin-Bordes C, Berger P, Taille CA, et al. proof of concept randomized-controlled trial of omalizumab in patients with severe difficult to control nonatopic asthma. Chest. 2013;144:411–9. doi: 10.1378/chest.12-1961. [DOI] [PubMed] [Google Scholar]
- 20.Lommatzsch M, Korn S, Buhl R, Virchow JC. Against all odds: anti-IgE for intrinsic asthma? Thorax. 2014;69:94–6. doi: 10.1136/thoraxjnl-2013-203738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothenberg ME, Klion AD, Roufosse FE, Kahn JE, Weller PF, Simon HU, et al. Treatment of patients with the hypereosinophilic syndrome with mepolizumab. N Engl J Med. 2008;358:1215–28. doi: 10.1056/NEJMoa070812. [DOI] [PubMed] [Google Scholar]
- 22.Haldar P, Brightling CE, Hargadon B, Gupta S, Monteiro W, Sousa A, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360:973–84. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nair P, Pizzichini MM, Kjarsgaard M, Inman MD, Efthimiadis A, Pizzichini E, et al. Mepolizumab for prednisone-dependent asthma with sputum eosinophilia. N Engl J Med. 2009;360:985–93. doi: 10.1056/NEJMoa0805435. [DOI] [PubMed] [Google Scholar]
- 24.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380:651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 25.Castro M, Mathur S, Hargreave F, Boulet LP, Xie F, Young J, et al. Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med. 2011;184:1125–32. doi: 10.1164/rccm.201103-0396OC. [DOI] [PubMed] [Google Scholar]
- 26.Laviolette M, Gossage DL, Gauvreau G, Leigh R, Olivenstein R, Katial R, et al. Effects of benralizumab on airway eosinophils in asthmatic patients with sputum eosinophilia. J Allergy Clin Immunol. 2013;132:1086–96. doi: 10.1016/j.jaci.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011;128:257–63. doi: 10.1016/j.jaci.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 28.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–98. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 29.Taube C, Duez C, Cui ZH, Takeda K, Rha YH, Park JW, et al. The role of IL-13 in established allergic airway disease. J Immunol. 2002;169:6482–9. doi: 10.4049/jimmunol.169.11.6482. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie AN, Fallon PG. Decoy receptors in the regulation of T helper cell type 2 responses. J Exp Med. 2003;197:675–9. doi: 10.1084/jem.20030096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–66. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 32.Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate- to-severe atopic dermatitis. N Engl J Med. 2014;371:130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 33.Gauvreau GM, Boulet LP, Cockcroft DW, FitzGerald JM, Mayers I, Carlsten C, et al. OX40L blockade and allergeninduced airway responses in subjects with mild asthma. Clin Exp Allergy. 2014;44:29–37. doi: 10.1111/cea.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–24. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brusselle GG, Vanderstichele C, Jordens P, Deman R, Slabbynck H, Ringoet V, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322–9. doi: 10.1136/thoraxjnl-2012-202698. [DOI] [PubMed] [Google Scholar]
- 36.Busse WW, Holgate S, Kerwin E, Chon Y, Feng J, Lin J, Lin SL. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med. 2013;188:12. doi: 10.1164/rccm.201212-2318OC. [DOI] [PubMed] [Google Scholar]


