Abstract
AIM: To assess the validity of our selection criteria for hepatectomy procedures based on indocyanine green disappearance rate (KICG), and to unveil the factors affecting posthepatectomy mortality in patients with hepatocellular carcinoma (HCC).
METHODS: A retrospective analysis of 198 consecutive patients with HCC who underwent partial hepatectomies in the past 14 years was conducted. The selection criteria for hepatectomy procedures during the study period were KICG ≥ 0.12 for hemihepatectomy, KICG ≥ 0.10 for bisegm-entectomy, KICG ≥ 0.08 for monosegmentectomy, and KICG ≥ 0.06 for nonanatomic hepatectomy. The hepatectomies were categorized into three types: major hepatectomy (hemihepatectomy or a more extensive procedure), bisegmentectomy, and limited hepatectomy. Univariate (Fisher’s exact test) and multivariate (the logistic regression model) analyses were used.
RESULTS: Postoperative mortality was 5% after major hepatectomy, 3% after bisegmentectomy, and 3% after limited hepatectomy. The three percentages were comparable (P = 0.876). The platelet count of ≤ 10104/mL was the strongest independent factor for postoperative mortality on univariate (P = 0.001) and multivariate (risk ratio, 12.5; P = 0.029) analyses. No patient with a platelet count of >7.3104/mL died of postoperative morbidity, whereas 25% (6/24 patients) of patients with a platelet count of ≤ 7.3104/mL died (P<0.001).
CONCLUSION: The selection criteria for hepatectomy procedures based on KICG are generally considered valid, because of the acceptable morbidity and mortality with these criteria. The preoperative platelet count independently affects morbidity and mortality after hepatectomy, suggesting that a combination of KICG and platelet count would further reduce postoperative mortality.
Keywords: Hepatocellular carcinoma, Hepatectomy, Morbidity, Mortality, Indocyanine green clearance test, Blood platelet count
INTRODUCTION
Postoperative morbidity and mortality remain a challenge following hepatectomy for hepatocellular carcinoma (HCC) of the injured liver[1-5]. Factors affecting morbidity and mortality after hepatectomy include hyperbilirubinemia[2,6] poor indocyanine green clearance[2-5,7] active hepatitis[1,2,5] low preoperative platelet count[6,8] hypoalbuminemia[8] high serum creatinine level[8] major hepatectomy[6,8] excessive blood loss during hepatectomy[4,6,9-11] and perioperative blood transfusion[8,12]. In our department, a hepatectomy procedure has been selected for each patient with HCC mainly based on indocyanine green disappearance rate. Despite this selection method, however, some patients have died of postoperative morbidity.
The aims of this study were to assess the validity of our selection criteria for hepatectomy procedures based on indocyanine green disappearance rate, and to unveil the risk factors for postoperative morbidity and mortality, in order to supplement the selection criteria for hepatectomy procedures for HCC.
MATERIALS AND METHODS
Patient population
From January 1990 to March 2004, 210 consecutive patients underwent partial hepatectomies for HCC in the study department. Eleven patients with concomitant primary malignant tumors in other organs and one with combined hepatocellular and cholangiocarcinoma were excluded. The remaining 198 patients formed the basis of this retrospective study, which included 145 men and 53 women with a median age of 65 years (range: 16-81 years). All patients were Japanese.
Hepatectomy procedures
In the study department, hepatectomy is the standard treatment for HCC whenever the tumors are considered to be resectable and the patient’s condition permits the resection. The hepatectomy procedures examined in this study included nonanatomic hepatectomy (removal of the tumor with a rim of non-neoplastic liver parenchyma) in 66 patients, anatomic monosegmentectomy (removal of 1 Couinaud’s segment[13]) in 34, anatomic bisegmentectomy (removal of two Couinaud’s segments) in 38, right hepatectomy (removal of Couinaud’s segments V-VIII) in 31, left hepatectomy (removal of Couinaud’s segments II-IV) in 6, extended right hepatectomy in 15, extended left hepatectomy in 6, and right trisectionectomy (removal of Couinaud’s segments IV-VIII) in 2. In the case of combined nonanatomic and anatomic hepatectomies in the same patient, the anatomic hepatectomy represented the hepatectomy procedure for that patient.
The hepatectomies were categorized into three types: major hepatectomy (hemihepatectomy or a more extensive procedure), bisegmentectomy, and limited hepatectomy (monosegmentectomy or nonanatomic hepatectomy). Intra-operative ultrasonography was employed for all patients. Lymph-node dissection of the hepatic hilum was not performed routinely, and only 7 patients underwent this type of dissection. The median operative time was 306 min (range: 100-730 min). The median estimated blood loss was 887 mL (range: 10-14 263 mL).
The selection criteria for hepatectomy procedures based on indocyanine green disappearance rate
In our department, the indocyanine green clearance test[14,15] has been routinely performed preoperatively, in order to assess hepatic functional reserve in patients for whom hepatectomies are planned. After the intravenous injection of indocyanine green (0.5 mg/kg, Diagnogreen; Daiichi Pharmaceutical Co., Inc., Tokyo, Japan), indocyanine green disappearance rate (KICG) was calculated by linear regression from the plasma indocyanine green concentrations at 5, 10, and 15 min. For the current series, the median indocyanine green retention rate at 15 min was 14% (range: 1-47%; reference range: 10% or less), whereas the median KICG was 0.136 (range: 0.04-0.243).
The indocyanine green clearance test is useful for estimating hepatic functional reserve in patients with injured livers[2-5,7]. In our department, the selection of a hepatectomy procedure for each patient has depended mainly on KICG. The selection criteria for this series were KICG ≥ 0.12 for hemihepatectomy, KICG ≥ 0.10 for bisegmentectomy, KICG ≥ 0.08 for monosegmentectomy, and KICG ≥ 0.06 for nonanatomic hepatectomy (including the enucleation of hepatic tumors).
Laboratory tests before hepatectomy
All patients underwent the following laboratory tests about 1 wk before hepatectomy: blood platelet count (reference range: 16.4-35.4104/mL), serum aspartate aminotransferase (reference range: 8-25 IU/L), serum alanine aminotransferase (reference range: 3-23 IU/L), serum total bilirubin (reference range: 3-9 mg/L), serum albumin (reference range: 41-50 g/L), blood urea nitrogen (reference range: 80-200 mg/L), serum creatinine (reference range: 5-8 mg/L), and prothrombin time (reference range: 81-131%).
Hepatitis B surface antigen and hepatitis C antibody in the serum were detected by radioimmunoassay (Lumipulse II HBsAg; Fujirebio Co., Inc., Tokyo, Japan) and a second-generation ELISA (Lumipulse II Ortho HCV; Ortho-Clinical Diagnostics Co., Inc., Tokyo, Japan), respectively. In the current series, 49 patients tested positive for hepatitis B surface antigen, 100 tested positive for hepatitis C antibody, and 2 tested positive for both. The remaining 47 patients tested negative for both.
Pathologic examination
The resected specimens were submitted to the Department of Surgical Pathology in our hospital for histologic evaluation. Each specimen was examined grossly and microscopically. The cancer stage was determined according to the pathologic tumor-node-metastasis (pTNM) staging system[16]. Cirrhosis in the resected liver was diagnosed microscopically based on the presence of fibrous septa and regenerative nodules. One hundred and six patients were found to be cirrhotic.
Definitions of postoperative morbidity and mortality
Postoperative morbidity was defined as any postoperative complication that lengthened the hospital stay, and included intra-abdominal bleeding (either interventional radiologic technique or reoperation required), hyperbilirubinemia (serum total bilirubin >50 mg/L, persisting for more than 7 d), hepatic encephalopathy, intractable ascites (abdominocentesis required), intractable pleural effusion (either insertion of a thoracic tube or two or more thoracocenteses required), biliary fistula, gastrointestinal hemorrhage, portal vein thrombosis, bowel obstruction (reoperation required), acute renal insufficiency (hemodialysis required), adult respiratory distress syndrome, sepsis, intra-abdominal infection, methicillin-resistantStaphylococcus aureus or pseudomembranous enterocolitis, pneumonia, and wound infection. Intra-abdominal infection was diagnosed when either an abscess was detected on imaging or positive bacterial culture of a drainage fluid was identified together with a fever (> 38 °C) without extra-abdominal septic complications. Tumor progression during the hospital stay (one patient) was not treated as a postoperative complication.
Postoperative mortality was defined as any death occurring during the hospital stay for resection of HCC.
Risk factors for postoperative morbidity and mortality
All 198 patients were tested for a total of 20 factors which might influence postoperative morbidity and mortality[17]: age ( ≤ 65 years vs >65 years), gender, the Child-Pugh classification[18] (A vs B+C), cirrhosis (absent vs present), hepatitis B viral status (positive vs negative for hepatitis B surface antigen), hepatitis C viral status (positive vs negative for hepatitis C antibody), blood platelet count ( ≤ 10104/mL vs >10104/mL), serum aspartate aminotransferase ( ≤ 50 IU/L vs >50 IU/L), serum alanine aminotransferase ( ≤ 46 IU/L vs > 46 IU/L), serum total bilirubin ( ≤ 10mg/L vs >10mg/L), serum albumin ( ≤ 35 g/L vs > 35 g/L), blood urea nitrogen ( ≤ 200 mg/L vs >200 mg/L), serum creatinine ( ≤ 10 mg/L vs > 10 mg/L), prothrombin time ( ≤ 70% vs>70%), KICG ( ≤ 0.12 vs > 0.12), the type of hepatectomy (major hepatectomy vs bisegmentectomy vs limited hepatectomy), operative time ( ≤ 300 min vs > 300 min), estimated blood loss ( ≤ 800 mL vs >800 mL), blood transfusion (transfusion of packed red cells during the operation) (no vs yes), and pTNM stage[16] (I vs II+III+IV).
Statistical analysis
Medical records were obtained for all patients. Univariate analyses of risk factors for postoperative morbidity and mortality were performed using Fisher’s exact test. Multivariate analyses of the risk factors for postoperative morbidity and mortality were performed using the logistic regression model. In this model, a stepwise selection was used for the variable selection, with entry and removal limits of P < 0.1 and P > 0.15, respectively. The stability of this model was confirmed using a step-backward and step-forward fitting procedure; the variables identified as having an independent influence on morbidity and mortality were identical with both procedures. The Mann-Whitney test was used to compare the blood platelet counts between patients with postoperative mortality and patients without postoperative mortality. All statistical evaluations were performed using the SPSS 11.5J software package (SPSS Japan Inc., Tokyo, Japan). All tests were two-sided, and the differences with Pvalues lesser than 0.05 were considered statistically significant.
RESULTS
Postoperative complications occurred in 50 patients out of a total of 198 patients, resulting in a postoperative morbidity of 25%. Intra-abdominal infection was the most common complication (n = 30), followed by intractable pleural effusion (n = 18), wound infection (n = 9), gastrointestinal hemorrhage (n = 9), methicillin-resistant Staphylococcus aureus or pseudo-membranous enterocolitis (n = 8), pneumonia (n = 7), biliary fistula (n = 7), hepatic encephalopathy (n = 5), intractable ascites (n = 5), hyperbilirubinemia (n = 3), portal vein thrombosis (n = 3), acute renal insufficiency (n = 2), bowel obstruction (n = 2), sepsis (n = 1), intra-abdominal bleeding (n = 1), and adult respiratory distress syndrome (n = 1).
Seven patients died during their hospital stay, which resulted in a postoperative mortality of 3.5%. Among the seven patients with postoperative mortality, six died of postoperative morbidity and the other died of tumor progression.
Postoperative morbidity and mortality according to the type of hepatectomy procedure
The incidence of postoperative morbidity was 28% (17/60 patients) after major hepatectomy, 32% (12/38 patients) after bisegmentectomy, and 21% (21/100 patients) after limited hepatectomy. The postoperative morbidity incidences for the three types of hepatectomy were comparable (P = 0.365).
The incidence of postoperative mortality was 5% (3/60 patients) after major hepatectomy, 3% (1/38 patients) after bisegmentectomy, and 3% (3/100 patients) after limited hepatectomy. The postoperative mortality incidences for the three types of hepatectomy were comparable (P = 0.876).
Risk factors for postoperative morbidity
Univariate analysis revealed that the estimated blood loss (P<0.001), blood transfusion (P<0.001), operative time (P<0.001), and blood platelet count (P = 0.022) were significantly associated with postoperative morbidity. These four variables entered into multivariate analysis, and the estimated blood loss (risk ratio: 3.935; P = 0.003), blood transfusion (risk ratio: 3.704; P = 0.004), and blood platelet count (risk ratio: 3.069; P = 0.010) remained as significantly independent factors for postoperative morbidity (Table 1).
Table 1.
Risk factors for morbidity after hepatectomy
| Variable | Number of patients | Univariate analysis |
Multivariate analysis |
|||
| P | β | Risk ratio | 95%CI | P | ||
| Estimated blood loss (mL) | <0.001 | 1.370 | 3.935 | 1.578-9.814 | 0.003 | |
| ≤ 800 (n = 91) | 8 | |||||
| > 800 (n = 107) | 42 | |||||
| Blood transfusion | <0.001 | 1.309 | 3.704 | 1.520-9.009 | 0.004 | |
| No (n = 94) | 9 | |||||
| Yes (n = 104) | 41 | |||||
| Operative time (min) | <0.001 | - | - | - | - | |
| ≤ 300 (n=96) | 13 | |||||
| > 300 (n=102) | 37 | |||||
| Blood platelet count (×104/μL) | 0.022 | 1.043 | 3.069 | 1.287-6.250 | 0.01 | |
| ≤ 10 (n=49) | 19 | |||||
| > 10 (n=149) | 31 | |||||
CI: Confidence interval.
Risk factors for postoperative mortality
Univariate analysis revealed that the blood platelet count (P = 0.001), prothrombin time (P = 0.005), and Child-Pugh classification (P = 0.044) were significantly associated with postoperative mortality. These three variables entered into multivariate analysis, and the blood platelet count (risk ratio: 12.50; P = 0.029) remained as the only significantly independent factor for postoperative mortality (Table 2).
Table 2.
Risk factors for mortality after hepatectomy
| Variable | Number of patients | Univariate analysis |
Multivariate analysis |
|||
| P | β | Risk ratio | 95%CI | P | ||
| Blood platelet count (×104/μL) | 0.001 | 2.520 | 12.50 | 1.302–125.0 | 0.029 | |
| ≤ 10 (n = 49) | 6 | |||||
| > 10 (n = 149) | 1 | |||||
| Prothrombin time (%) | 0.005 | 1.476 | 4.367 | 0.812–23.81 | 0.086 | |
| ≤ 70 (n = 24) | 4 | |||||
| > 70 (n = 174) | 3 | |||||
| Child–Pugh classification | 0.044 | – | – | – | – | |
| A (n = 173) | 4 | |||||
| B+C (n = 25) | 3 | |||||
CI: Confidence interval.
Blood platelet count and postoperative mortality
The blood platelet counts were significantly lower in patients with postoperative mortality than in those without (the Mann-Whitney test, P = 0.003, Figure 1). Among the seven patients with postoperative mortality, the patient who died of tumor progression during the hospital stay showed a platelet count of 25.7×104/μL, whereas the other patients who died of postoperative morbidity showed platelet counts of 7.3×104/μL, 7.1×104/μL, 6.9×104/μL, 6.4×104/μL, 5.0×104/μL, and
Figure 1.
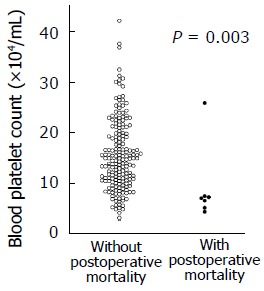
Patients with postoperative mortality had lower platelet counts than those without (the Mann-Whitney test, P = 0.003). The platelet counts were 8.9 ± 7.5 × 104/μL (mean±SD) in patients with postoperative mortality, whereas they were 15.4 ± 7.0 × 104/μL (mean±SD) in those without postoperative mortality. 1This patients howed a platelet count of 25.7 × 104/μL and died of tumor progression, and not of postoperative morbidity, during the hospital stay.
4.2×104/μL. Thus, no patient with a platelet count of > 7.3×104/μL died of postoperative morbidity, whereas the postoperative mortality was 25% (6/24 patients) among patients with platelet counts of ≤ 7.3×104/μL (P < 0.001).
DISCUSSION
The indocyanine green clearance test has been reported to provide a reliable selection criterion for hepatectomy procedures for patients with resectable HCCs[2-4]. In the current series, a hepatectomy procedure was selected for each patient mainly based on KICG. As a result, both the postoperative morbidities and mortalities became comparable among the three types of hepatectomy. Although major hepatectomy is considered to be a high-risk treatment option for HCC patients with poor hepatic functional reserves[7,10,19] in our patients it resulted in an acceptable morbidity (28%) and mortality (5%). The above suggests that our selection criteria for hepatectomy procedures using KICG are generally valid. We regret, however, that some patients died of postoperative complications in spite of these selection criteria for hepatectomy procedures. This has prompted us to conduct the current study.
A low preoperative platelet count is related to portal hypertension and its resulting hypersplenism, hepatic fibrosis, and unfavorable indocyanine green clearance test results[20-22]. Jarnagin et al[6] and Poon et al[8] have documented the fact that a low preoperative platelet count is associated with a higher incidence of postoperative mortality. Bennett and Blumgart have stated,“We are cautious when planning resections in patients with counts less than 100 000/mL”[23]. Poon and Fan have also affirmed that cirrhotic patients with platelet counts of less than 10104/mL should not be considered for major hepatectomy[24]. Among various factors affecting perioperative mortality, the preoperative platelet count is clinically relevant, because it is readily available before surgery.
In this series, a hepatectomy procedure was selected for each patient based on KICG values, and a low preoperative platelet count ( ≤ 10104/mL) was the strongest independent factor for postoperative mortality; no patient with a count of > 7.3104/mL died of postoperative complications, whereas the mortality was high (25%) among those with counts of ≤ 7.3104/mL. This suggests that the combined use of KICG and the preoperative platelet count would further reduce postoperative mortality in patients with HCC.
The estimated blood loss and blood transfusion also independently affected the postoperative morbidity in the current series. Recent evidence suggests that blood loss is associated with increased postoperative morbidity and mortality[4,6,9-11]. Blood transfusion is also related to the recurrence of HCC[25-27]. In order to reduce blood loss, adequate and wide exposure of the operative field is essential[25]. During parenchymal transection, intermittent use of the Pringle maneuver[28,29] the use of developed modalities[30-33] and a lowering of the central venous pressure[34-37] are important. Meticulous surgical techniques are required to minimize blood loss. Only experienced surgeons should perform hepatectomies for HCC of cirrhotic liver.
The current study has limitations, such as the retrospective nature of the study and the small number of patients. However, we feel that these limitations did not significantly influence the outcome of the study, because the differences between the groups were too marked to have resulted from these biases.
In conclusion, we believe that the selection criteria for hepatectomy procedures based on KICG are generally valid because of the acceptable morbidity and mortality with these criteria. The preoperative platelet count independently affects morbidity and mortality after hepatectomy, suggesting that a combination of KICG and the platelet count would further reduce postoperative mortality in patients with HCC.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Noun R, Jagot P, Farges O, Sauvanet A, Belghiti J. High preoperative serum alanine transferase levels: effect on the risk of liver resection in Child grade A cirrhotic patients. World J Surg. 1997;21:390–394; discussion 395. doi: 10.1007/pl00012259. [DOI] [PubMed] [Google Scholar]
- 2.Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255–1259. [PubMed] [Google Scholar]
- 3.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg. 1992;163:515–518. doi: 10.1016/0002-9610(92)90400-l. [DOI] [PubMed] [Google Scholar]
- 4.Nonami T, Nakao A, Kurokawa T, Inagaki H, Matsushita Y, Sakamoto J, Takagi H. Blood loss and ICG clearance as best prognostic markers of post-hepatectomy liver failure. Hepatogastroenterology. 1999;46:1669–1672. [PubMed] [Google Scholar]
- 5.Eguchi H, Umeshita K, Sakon M, Nagano H, Ito Y, Kishimoto SI, Dono K, Nakamori S, Takeda T, Gotoh M, et al. Presence of active hepatitis associated with liver cirrhosis is a risk factor for mortality caused by posthepatectomy liver failure. Dig Dis Sci. 2000;45:1383–1388. doi: 10.1023/a:1005564205755. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406; discussion 406-7. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan ST, Lai EC, Lo CM, Ng IO, Wong J. Hospital mortality of major hepatectomy for hepatocellular carcinoma associated with cirrhosis. Arch Surg. 1995;130:198–203. doi: 10.1001/archsurg.1995.01430020088017. [DOI] [PubMed] [Google Scholar]
- 8.Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698–708; discussion 708-10. doi: 10.1097/01.sla.0000141195.66155.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagao T, Inoue S, Mizuta T, Saito H, Kawano N, Morioka Y. One hundred hepatic resections. Indications and operative results. Ann Surg. 1985;202:42–49. doi: 10.1097/00000658-198507000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33–40. doi: 10.1097/00000658-198701000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada M, Matsumata T, Akazawa K, Kamakura T, Itasaka H, Sugimachi K, Nose Y. Estimation of risk of major complications after hepatic resection. Am J Surg. 1994;167:399–403. doi: 10.1016/0002-9610(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 12.Gozzetti G, Mazziotti A, Grazi GL, Jovine E, Gallucci A, Gruttadauria S, Frena A, Morganti M, Ercolani G, Masetti M. Liver resection without blood transfusion. Br J Surg. 1995;82:1105–1110. doi: 10.1002/bjs.1800820833. [DOI] [PubMed] [Google Scholar]
- 13.Couinaud C. Le foie. Etudes anatomiques et chirurgicales. Paris: Masson; 1957. [Google Scholar]
- 14.Kawasaki S, Sugiyama Y, Iga T, Hanano M, Sanjo K, Beppu T, Idezuki Y. Pharmacokinetic study on the hepatic uptake of indocyanine green in cirrhotic patients. Am J Gastroenterol. 1985;80:801–806. [PubMed] [Google Scholar]
- 15.Moody FG, Rikkers LF, Aldrete JS. Estimation of the functional reserve of human liver. Ann Surg. 1974;180:592–598. doi: 10.1097/00000658-197410000-00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. American Joint Committee on cancer staging manual. 6th ed. Springer Verlag. 2002. pp. 131–138. [Google Scholar]
- 17.Wei AC, Tung-Ping Poon R, Fan ST, Wong J. Risk factors for perioperative morbidity and mortality after extended hepatectomy for hepatocellular carcinoma. Br J Surg. 2003;90:33–41. doi: 10.1002/bjs.4018. [DOI] [PubMed] [Google Scholar]
- 18.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 19.Matsumata T, Kanematsu T, Shirabe K, Sonoda T, Furuta T, Sugimachi K. Decreased morbidity and mortality rates in surgical patients with hepatocellular carcinoma. Br J Surg. 1990;77:677–680. doi: 10.1002/bjs.1800770629. [DOI] [PubMed] [Google Scholar]
- 20.Shimada M, Matsumata T, Adachi E, Itasaka H, Watiyama S, Sugimachi K. Estimation of degree of liver cirrhosis using a fibrosis score; a multivariate analysis of clinical parameters and resected specimens. Hepatogastroenterology. 1994;41:177–180. [PubMed] [Google Scholar]
- 21.Kajiwara E, Akagi K, Azuma K, Onoyama K, Fujishima M. Evidence for an immunological pathogenesis of thrombocytopenia in chronic liver disease. Am J Gastroenterol. 1995;90:962–966. [PubMed] [Google Scholar]
- 22.Kusaka K, Harihara Y, Torzilli G, Kubota K, Takayama T, Makuuchi M, Mori M, Omata S. Objective evaluation of liver consistency to estimate hepatic fibrosis and functional reserve for hepatectomy. J Am Coll Surg. 2000;191:47–53. doi: 10.1016/s1072-7515(00)00309-4. [DOI] [PubMed] [Google Scholar]
- 23.Bennett JJ, Blumgart LH. Assessment of hepatic reserve prior to hepatic resection. J Hepatobiliary Pancreat Surg. 2005;12:10–15. doi: 10.1007/s00534-004-0950-3. [DOI] [PubMed] [Google Scholar]
- 24.Poon RT, Fan ST. Assessment of hepatic reserve for indication of hepatic resection: how I do it. J Hepatobiliary Pancreat Surg. 2005;12:31–37. doi: 10.1007/s00534-004-0945-0. [DOI] [PubMed] [Google Scholar]
- 25.Fan ST, Ng IO, Poon RT, Lo CM, Liu CL, Wong J. Hepatectomy for hepatocellular carcinoma: the surgeon's role in long-term survival. Arch Surg. 1999;134:1124–1130. doi: 10.1001/archsurg.134.10.1124. [DOI] [PubMed] [Google Scholar]
- 26.Matsumata T, Ikeda Y, Hayashi H, Kamakura T, Taketomi A, Sugimachi K. The association between transfusion and cancer-free survival after curative resection for hepatocellular carcinoma. Cancer. 1993;72:1866–1871. doi: 10.1002/1097-0142(19930915)72:6<1866::aid-cncr2820720613>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, Yamaguchi N, Mizuno S, Makuuchi M. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 28.Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704–11; discussion 711-3. doi: 10.1097/00000658-199712000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li SQ, Liang LJ, Huang JF, Li Z. Ischemic preconditioning protects liver from hepatectomy under hepatic inflow occlusion for hepatocellular carcinoma patients with cirrhosis. World J Gastroenterol. 2004;10:2580–2584. doi: 10.3748/wjg.v10.i17.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto Y, Ikai I, Kume M, Sakai Y, Yamauchi A, Shinohara H, Morimoto T, Shimahara Y, Yamamoto M, Yamaoka Y. New simple technique for hepatic parenchymal resection using a Cavitron Ultrasonic Surgical Aspirator and bipolar cautery equipped with a channel for water dripping. World J Surg. 1999;23:1032–1037. doi: 10.1007/s002689900619. [DOI] [PubMed] [Google Scholar]
- 31.Sugo H, Mikami Y, Matsumoto F, Tsumura H, Watanabe Y, Kojima K, Futagawa S. Hepatic resection using the harmonic scalpel. Surg Today. 2000;30:959–962. doi: 10.1007/s005950070055. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Sasaki Y, Yokoyama S, Miyashiro I, Murata K, Doki Y, Kameyama M, Ohigashi H, Hiratsuka M, Ishikawa O, et al. Practical usefulness of bipolar scissors in hepatectomy. Hepatogastroenterology. 2002;49:597–600. [PubMed] [Google Scholar]
- 33.Wu W, Lin XB, Qian JM, Ji ZL, Jiang Z. Ultrasonic aspiration hepatectomy for 136 patients with hepatocellular carcinoma. World J Gastroenterol. 2002;8:763–765. doi: 10.3748/wjg.v8.i4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg. 1998;85:1058–1060. doi: 10.1046/j.1365-2168.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 35.Melendez JA, Arslan V, Fischer ME, Wuest D, Jarnagin WR, Fong Y, Blumgart LH. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg. 1998;187:620–625. doi: 10.1016/s1072-7515(98)00240-3. [DOI] [PubMed] [Google Scholar]
- 36.Itamoto T, Katayama K, Nakahara H, Tashiro H, Asahara T. Autologous blood storage before hepatectomy for hepatocellular carcinoma with underlying liver disease. Br J Surg. 2003;90:23–28. doi: 10.1002/bjs.4012. [DOI] [PubMed] [Google Scholar]
- 37.Smyrniotis V, Kostopanagiotou G, Theodoraki K, Tsantoulas D, Contis JC. The role of central venous pressure and type of vascular control in blood loss during major liver resections. Am J Surg. 2004;187:398–402. doi: 10.1016/j.amjsurg.2003.12.001. [DOI] [PubMed] [Google Scholar]


