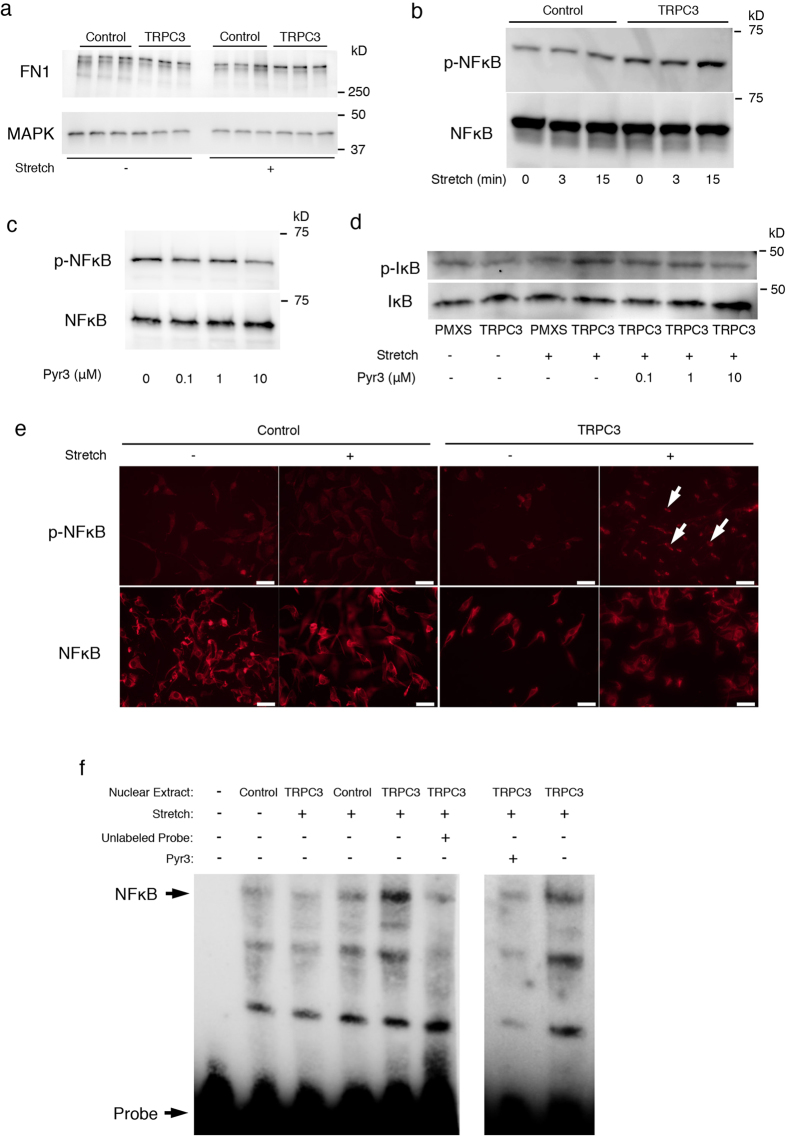
Figure 5. TRPC3 mediates cyclic stretching forces via NFκB activation in fibroblasts.
To elucidate the mechanism by which Fibronectin expression is increased in TRPC3 overexpressing cells in response to mechanical stretching, the activity of transcriptional factor NFκB was assessed. To clarify the data, the blots were cropped. Uncropped, full-length blots for Western Blot and EMSA are presented in Supplementary Figures S11–20. a; In order to determine whether NFκB is involved in the regulation of Fibronectin production, TRPC3 overexpressing cells and control cells were stretched (20%, 10 Hz) with/without an NFκB inhibitor (Wedelolactone) for 24 hours. Western blot analysis demonstrated that the increased expression of Fibronectin in TRPC3 overexpressing cells in response to mechanical stretching was blocked by Wedelolactone. b; The phosphorylation of NFκB in TRPC3 overexpressing cells was upregulated in a time dependent manner compared to control cells c; The phosphorylation of NFκB in response to stretching stimuli was attenuated by the TRPC3 specific inhibitor, Pyr3. d; The phosphorylation pattern of IκB, which is a regulator of NFκB activation, was assessed. TPC3 overexpressing fibroblasts showed more activation of IκB in response to mechanical stretching compared to control fibroblasts. The activation of IκB was attenuated by Pyr3 in dose dependent manner. e; Translocation of phosphorylated NFκB was evident in TRPC3 overexpressing fibroblasts stretched for 15 minutes. Arrows indicate translocated phosphorylated NFκB. Scale bars for each images = 50 μm. f; TRPC3 overexpressing fibroblasts and control fibroblasts were stretched for 15 minutes and the nuclear proteins were extracted. A gel shift assay was then performed, as described in the Materials and Methods section. Gel shift was most apparent in stretched TRPC3 overexpressing fibroblasts, which was attenuated by the addition of Pyr3.