Abstract
Parapharyngeal space (PPS) tumors are rare and account for about 0.5% of all head and neck neoplasms. Most PPS tumors are benign (up to 80%) while the remaining 20% are malignant. These tumors are either primaries; most commonly arising from salivary glands or metastatic tumors or due to direct extension of tumors from the adjacent sites. Distant metastasis from breast cancers more commonly involves the lungs, bones, brain and liver. Metastasis to the PPS from a primary breast carcinoma is rare, with only one case reported in literature. We, to the best of our knowledge report the second case of a carcinoma breast metastasizing to the PPS and further discuss the diagnostic and therapeutic challenges involved in its management. A fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography scan apart from explicitly defining the extent of the PPS tumor, majorly influenced the therapeutic decision making process by ruling out other sites of metastasis.
Keywords: Fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography, malignant salivary gland tumors, metastatic breast carcinoma, parapharyngeal space tumors
INTRODUCTION
The parapharyngeal space (PPS) is one of the potential spaces surrounding the pharynx. The neoplasms of PPS are rare and account for about 0.5% of all head and neck neoplasms.[1] A majority of the PPS tumors are benign (up to 80%) while the remaining 20% are malignant.[2] These tumors are either primaries-most commonly arising from salivary glands, or metastatic or direct extension of tumors from the adjacent sites.[3,4] Metastasis to the PPS from a primary breast carcinoma is rare, with only one case reported in literature.[5] We possibly present the second case of carcinoma breast metastasizing to PPS after a disease free interval of 7 years.
CASE REPORT
A 60-year-old lady with no co-morbid illnesses presented to our center with a painless swelling in the right side of the upper neck of 6 months duration. Her past history included treatment for carcinoma right breast at our center 7 years prior. (T3N1M0 for which she received multimodality treatment consisting of surgery, chemotherapy and radiotherapy) The histopathology of the mastectomy specimen revealed an infiltrating ductal carcinoma and mucinous carcinoma. The tumor cells showed strong positivity for estrogen receptor (ER) and progesterone receptor (PR) and the patient had received adjuvant aromatase inhibitor (letrozole 2.5 mg) for 5 years and was on regular follow-up.
Clinical examination revealed a large well circumscribed, firm swelling measuring 6 cm × 5 cm in the right side of the upper neck. Examination of the cervical lymph nodes and the upper aero-digestive tract was normal and so were the examinations of the left breast, right chest wall, both axilla and supraclavicular fossa. Fine needle aspiration cytology from the mass was reported as poorly differentiated carcinoma.
A fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) done as a part of the staging work up revealed an well enhancing irregular lobulated soft tissue mass measuring 6 cm × 5 cm × 4 cm arising from the right PPS in relation to the deep lobe of the right parotid gland, extending from the level of the cervical vertebra C1 to C5, causing mass effect on the major neck vessels, that is, infiltrating the right internal jugular vein and displacing the right carotid vessels without displacing it (SUVmax 9.1). There was no other significant uptake on the PET-CT [Figure 1a–d].
Figure 1.
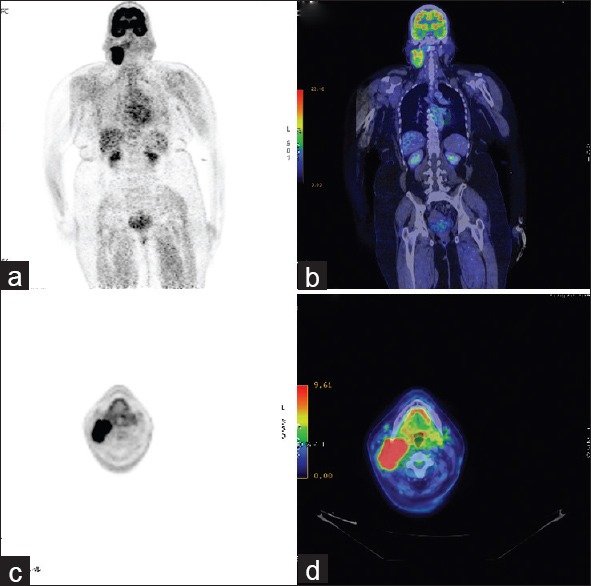
(a-d) Coronal (a and b) and axial (c and d) views of fluorine-18 fluorodeoxyglucose positron emission tomography (18F-FDG PET) and the corresponding 18F-FDG PET-computed tomography images showing the isolated well enhancing (SUVmax 9.1) irregular lobulated soft tissue mass arising from the right parapharyngeal space in relation to the deep lobe of the right parotid gland, extending from the level of the cervical vertebra C1–C5, causing mass effect on the major neck vessels
The provisional diagnosis was that of a malignant salivary gland carcinoma involving the PPS. A rare possibility of a parapharyngeal metastasis from breast carcinoma was also considered in view of the prior history of breast cancer.
It was felt that surgery would still be the treatment of choice even in the later scenario; considering the long disease free interval and the solitary site of metastasis. The patient was therefore planned for a definitive radical surgery. Intra-operatively the mass was dissected out from the surrounding structures and was removed en bloc along with the right internal jugular vein and the neck nodes; resulting in a formal radical neck dissection [Figure 2a and b], The patient made an uneventful postoperative recovery. Microscopically, the tumor cells were arranged predominantly as nests and islands with few foci of tubule formation, separated by scanty stroma. They were round to polygonal, with moderate eosinophilic cytoplasm, vesicular nuclei with fine chromatin [Figure 3a]. Increased mitosis and vascular invasion was also seen. On immunohistochemistry (IHC), the tumour cells showed positivity for PR, ER, [Figure 3b], pan-keratin and high molecular weight keratin [Figure 3c]. The Ki-67 proliferative index was 50–60% [Figure 3d]. Further the tumour cells were positive for the neuroendocrine markers CD56, neuron-specific enolase, synatophysin [Figure 3e], chromogranin [Figure 3f]. The tumour cells showed negative reaction to C-kit, gross cystic disease fluid protein-15. Based on the histopathological and IHC findings, the tumour was finally diagnosed as metastatic carcinoma of breast with neuroendocrine differentiation.
Figure 2.
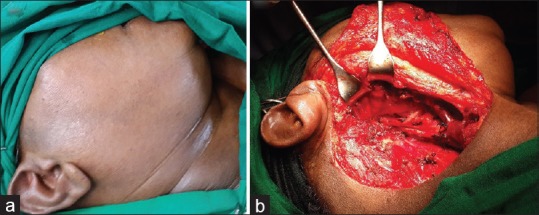
(a) Preoperative clinical photograph. (b) Intra-operative clinical photograph following the removal of the right parapharyngeal tumor
Figure 3.
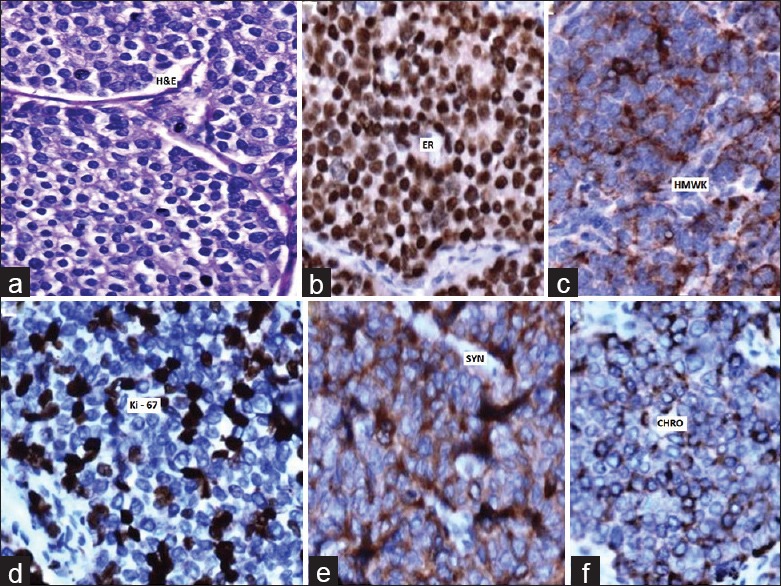
(a) H and E, ×20 microphotograph showing the tumor cells arranged as nests and islands with few foci of tubule formation, separated by scanty stroma epithelial cell islands. (b) Immunohistochemistry (IHC), ×20 tumor cells showing strong positivity for estrogen receptors. (c) IHC, ×20 tumor cells showing positivity for high molecular weight keratin. (d) IHC, ×20 tumor cells showing positivity for Ki-67. (e) IHC, ×20 tumor cells showing positivity for synatophysin. (f) IHC, ×20 tumor cells showing positivity for chromogranin
The patient after a multidisciplinary board discussion was subsequently considered for external beam radiotherapy to the tumor bed and is further planned for a second line chemotherapy followed by hormonal therapy.
DISCUSSION
Distant metastasis from breast cancers typically involves the lungs, bones, brain, and liver.[6] Breast carcinoma metastases to the head and neck region is very rare,[7] however isolated case reports of metastasis in the larynx, nasopharynx, parotid gland, nose; paranasal sinuses and temporal bone have been periodically reported.
Studies have reported differing patterns of metastatic spread between invasive ductal and invasive lobular breast carcinomas, the reasons for which is not completely known.[6] Lobular carcinomas are in fact more likely to metastasize to unusual sites like the gastrointestinal tract.[6] Metastasis to the PPS from a primary breast cancer is extraordinarily rare and to the best of knowledge, ours is the second case and interestingly of ductal origin.
Although the history and physical examination can provide clues to the site of origin and nature of a PPS tumor, imaging studies are more useful for defining the site of origin and extent of the mass, as well as its vascularity and relationship to the great vessels of the neck and other neurovascular structures. An 18F-FDG PET/CT scan in our case, apart from explicitly defining the extent of the PPS tumor, further aided the decision to go ahead with a radical surgery by ruling out further sites of distant metastasis.
The accurate diagnosis in this present case was a pathological challenge because of the following two reasons: Firstly, the common tumors in PPS include benign or malignant neoplasms arising from salivary glands, while metastasis from a carcinoma breast is a rare differential.[5] Since the tumour in our patient showed neuroendocrine features, solid variant of adenoid cystic carcinoma was additionally considered as a differential diagnosis. However, negative reaction to C-kit in this tumour ruled out the diagnosis of primary salivary gland adenoid cystic carcinoma.[8]
Secondly, the primary breast tumour showed features of a combined infiltrating ductal and mucinous carcinoma, whereas the metastatic tumour showed morphological features of a neuroendocrine carcinoma. This could be explained on account of the pathogenesis of breast neuroendocrine tumors. The existence of neuroendocrine differentiation in the mammary tissue is a well-known entity, contemporary thinking has however suggested that neuroendocrine tumors of the breast does not constitute a single clinico-pathological entity with a consistent histogenesis but rather represents a pathway of neoplastic development available to a range of breast cancers including ductal carcinoma in situ, mucinous carcinoma, a variant of lobular carcinoma and even low grade invasive ductal carcinoma. Features of neuroendocrine differentiation are detectable in carcinomas of the breast either as scattered cells immune-reactive to the neuroendocrine markers or as special type of tumors wherein the vast majority of the cells display neuroendocrine characteristics.[9]
Surgery is the mainstay of management of primary malignant PPS tumors.[1,2,3,4] The choice of treatment for metastatic tumors of the PPS however is extremely difficult due to paucity of data and needs to be individualized as was done in our patient.[10]
CONCLUSION
Our case reiterates the importance of maintaining a high index of suspicion for metastatic disease in patients with a prior history of invasive cancer. A 18F-FDG PET/CT scan, by ruling out other sites of metastasis can majorly influence the therapeutic decision making process in the management of metastatic tumors of the PPS, as was seen in our patient.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Krishnamurthy A, Ramshankar V, Majhi U. A large extra cranial cystic trigeminal schwannoma of the parapharyngeal space-Exploring the right approach. Indian J Surg Oncol. 2014;5:196–8. doi: 10.1007/s13193-014-0326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olsen KD. Tumors and surgery of the parapharyngeal space. Laryngoscope. 1994;104:1–28. doi: 10.1288/00005537-199405000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Khafif A, Segev Y, Kaplan DM, Gil Z, Fliss DM. Surgical management of parapharyngeal space tumors: A 10-year review. Otolaryngol Head Neck Surg. 2005;132:401–6. doi: 10.1016/j.otohns.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 4.Bozza F, Vigili MG, Ruscito P, Marzetti A, Marzetti F. Surgical management of parapharyngeal space tumours: Results of 10-year follow-up. Acta Otorhinolaryngol Ital. 2009;29:10–5. [PMC free article] [PubMed] [Google Scholar]
- 5.Raut V, Sinnathuray AR, McClean G, Brooker D. Metastatic breast carcinoma in the parapharyngeal space. J Laryngol Otol. 2001;115:750–2. doi: 10.1258/0022215011908847. [DOI] [PubMed] [Google Scholar]
- 6.Gujral DM, Quante M, Simcock RA. An unusual cause of dysphagia in ductal breast cancer due to submucosal oropharyngeal metastatic spread: A case report. Cases J. 2009;2:3. doi: 10.1186/1757-1626-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuler PJ, Heikaus S, Friebe-Hoffmann U, Hoffmann TK, Greve J, Klenzner T, et al. Breast cancer metastases in the head and neck region. HNO. 2010;58:859–65. doi: 10.1007/s00106-010-2150-6. [DOI] [PubMed] [Google Scholar]
- 8.Dabbs J. 4th ed. Philadelphia, PA: Elsevier/Saunders; 2014. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. [Google Scholar]
- 9.Sapino A, Righi L, Cassoni P, Papotti M, Pietribiasi F, Bussolati G. Expression of the neuroendocrine phenotype in carcinomas of the breast. Semin Diagn Pathol. 2000;17:127–37. [PubMed] [Google Scholar]
- 10.Pagani O, Senkus E, Wood W, Colleoni M, Cufer T, Kyriakides S, et al. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J Natl Cancer Inst. 2010;102:456–63. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]


