Abstract
Patients with Marfan syndrome (MFS) presents with primary skeletal manifestations such as tall stature, chest wall abnormality, and scoliosis. These primary skeletal manifestations affect the growth pattern in MFS. Therefore, it is not appropriate to use normal growth charts to evaluate the growth status of MFS. We aimed to develop disease-specific growth charts for Korean MFS patients and to use these growth charts for understanding the growth patterns in MFS and managing of patients with MFS. Anthropometric data were available from 187 males and 152 females with MFS through a retrospective review of medical records. Disease-specific growth charts were generated and 3, 25, 50, 75, and 97 percentiles were calculated using the LMS (refers to λ, μ, and σ, respectively) smoothing procedure for height and weight. Comparisons between MFS patients and the general population were performed using a one-sample t-test. With regard to the height, the 50th percentile of MFS is above the normative 97th percentile in both genders. With regard to the weight, the 50 percentile of MFS is above the normative 75th percentile in male and between the normative 50th percentile and the 75th percentile in female. The disease-specific growth charts for Korean patients with MFS can be useful for monitoring growth patterns, planning the timing of growth-reductive therapy, predicting adult height and recording responses to growth-reductive therapy.
Keywords: Marfan Syndrome, Growth Charts, Body Height, Body Weight
INTRODUCTION
Marfan syndrome (MFS; MIM# 154700) is an autosomal dominantly inherited disorder caused by mutations in the fibrillin-1 (FBN1) gene located on chromosome 15q21 that typically affects the cardiovascular, skeletal, and ocular systems (1, 2, 3). The incidence of MFS is approximately 1 per 10,000 individuals, and it does not show geographic, ethnic, or gender differences (3, 4). Although the clinical manifestations of MFS are highly diverse within affected families, the primary skeletal manifestations, such as tall stature, chest wall abnormality, and scoliosis are relatively common characteristics of MFS. And these primary skeletal manifestations affect the growth pattern in MFS.
Growth-reductive therapy with high dose sex steroids is a well-known, though still controversial, therapy. Due to the different growth pattern of MFS and the effect of growth-reductive therapy before puberty, disease-specific growth charts for MFS are needed. It would be useful for understanding the growth pattern in MFS, for predicting adult height and for planning the timing of growth-reductive therapy. Recently, only one study has presented disease-specific growth charts for MFS (5). There have been some reports that the clinical phenotype of Korean and Japanese MFS patients may differ from that of Western populations (6, 7). Therefore, we have reviewed the growth parameters of patients with MFS in Korea and generated the Korean MFS-specific growth charts.
MATERIALS AND METHODS
Patients
Clinical data were collected retrospectively from a review of medical records of patients with clinically diagnosed MFS using the revised Ghent criteria (8) who visited the outpatient clinic at Samsung Medical Center between January 1995 and March 2013. Exclusion criteria for data collection were as follows: 1) Patients who had received sex steroid hormone therapy for growth reduction, 2) patients who had undergone epiphysiodesis or any other height-altering treatment to the growth plates. Three hundred and fifty patients have been diagnosed with MFS between January 1995 and March 2013. Of the 350 patients with clinically confirmed MFS according to the revised Ghent criteria (8), 9 patients had been treated with high dose estrogen therapy for growth reduction from the first measurements of their height and weight, and they were excluded from the analysis. Two patients were excluded from the analysis because their height and weight measurements were considered as outliers (more than 5 standard deviation scores from the mean). Anthropometric data were available on 339 patients (187 males and 152 females). The anthropometric measurements consisted of height and weight. The standing height measurements were obtained with an electronic stadiometer (DS-103, Dongsahn JENIX Co. Ltd, Seoul, Korea). Weight was measured with an electronic digital scale recorded to the nearest 0.01 kg (DS-B02, Dongsahn JENIX Co. Ltd).
Statistical analysis
Centile curves were constructed with the LMS Chart Maker Pro version 2.54 software program (The Institute of Child Health, London, UK), which fits smooth centile curves to reference data of the height and weight of the patients with MFS using the LMS method. This method summarizes percentiles at each age based on the power of age-specific Box-Cox power transformations that are used to normalize the data (10). The final curves of the percentiles were produced by three curves representing the skewness (L curves), the median (M curves), and the coefficient of variation (S curves). The L, M, and S values were smoothed for each age and gender using cubic spline curves (11).
Statistical analysis was performed using SPSS for Windows Version 21.0 (SPSS Inc., Chicago, IL, USA). All data were represented as the mean±standard deviation (SD) and number of subjects (percentage). Comparisons between the MFS patients and the general population were performed using a one-sample t-test. Significance was defined as P<0.05.
Ethics statement
This study was approved by the institutional review board of Samsung Medical Center (IRB No. SMC 2013-04-023). Informed consent was waived by the board because of the observational nature of the study.
RESULTS
Growth data were available from 187 males and 152 females with MFS through a retrospective review of medical records. The statistical analysis included 586 height measurements and 651 weight measurements for males and 596 height measurements and 665 weight measurements for females. The majority of anthropometric data (85.7% of the total height data, 86.1% of the total weight data) had longitudinal measurements. Approximately 4.2% of the data were available from birth to 36 months. For comparison with the general population, the 2007 Korean National Growth Chart was used (12).
Height growth curve
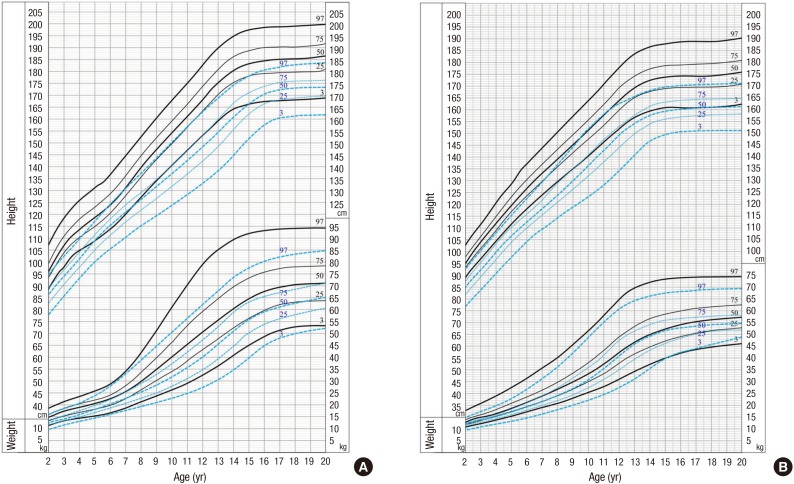
Up to the first year of age, the mean height of MFS patients tended to be similar to that of normal infants in both genders. During the next year, the mean height of MFS males had a tendency to be larger than normal (Table 1, 90.5±10.6 cm vs. 82.1±3.3 cm, P=0.109), and that of MFS females was significantly larger than normal (Table 2, 90.4±4.8 cm vs. 80.8±3.2 cm, P<0.001). After the second year of life, the mean height of MFS patients was larger than that of the general population in both genders. In males, the 3rd percentile of the MFS statural growth curve coincided with the 75th percentile of the standard curve by 4 yr of age, and it crossed the 50th percentile of the standard curve by 5 yr of age, and remained between the 25th and 75th percentile by 8 yr of age. Thereafter it coincided with the 75th percentile of the standard curve by 13 yr of age (Fig. 1). The mean MFS adult height was 189.8±4.4 cm, and it was above the 97th percentile for Korean adult males (184.2±5.9 cm, P<0.001).
Table 1. Anthropometric data of males with Marfan syndrome compared to the general population.
| Age (yr) | No. (Ht) | Mean Height±SD of MFS (cm) | Mean Height±SD of Normal (cm) | P | No. (Wt) | Mean Weight±SD of MFS (kg) | Mean Weight±SD of Normal (kg) | P |
|---|---|---|---|---|---|---|---|---|
| 0-1 | 14 | 60.7±12.1 | 67.6±2.6 | 0.077 | 18 | 5.5±2.7 | 8.0±0.9 | 0.01 |
| 1-2 | 6 | 90.5±10.6 | 82.1±3.3 | 0.109 | 6 | 13.0±2.5 | 11.3±1.2 | 0.162 |
| 2-3 | 6 | 100.8±3.0 | 91.0±4.0 | <0.001 | 5 | 15.6±0.4 | 13.5±1.4 | <0.001 |
| 3-4 | 36 | 113.7±6.3 | 98.2±4.1 | <0.001 | 38 | 18.1±2.0 | 15.3±1.6 | <0.001 |
| 4-5 | 26 | 116.1±5.6 | 105.2±4.2 | <0.001 | 26 | 19.8±3.1 | 17.4±1.9 | 0.001 |
| 5-6 | 31 | 122.8±6.8 | 111.8±4.4 | <0.001 | 32 | 20.7±3.2 | 19.7±2.2 | 0.076 |
| 6-7 | 36 | 128.4±5.9 | 117.9±4.7 | <0.001 | 39 | 24.0±3.7 | 22.1±2.6 | 0.004 |
| 7-8 | 37 | 137.0±5.1 | 123.7±5.1 | <0.001 | 43 | 27.4±4.0 | 24.8±3.3 | <0.001 |
| 8-9 | 46 | 145.0±8.1 | 129.1±5.5 | <0.001 | 48 | 33.8±7.2 | 27.8±4.2 | <0.001 |
| 9-10 | 35 | 151.7±7.5 | 134.2±5.9 | <0.001 | 36 | 37.5±9.0 | 31.3±5.3 | <0.001 |
| 10-11 | 41 | 158.9±7.6 | 139.4±6.3 | <0.001 | 45 | 44.7±12.7 | 35.5±6.5 | <0.001 |
| 11-12 | 36 | 164.6±7.6 | 145.3±6.9 | <0.001 | 45 | 50.7±12.7 | 40.3±7.7 | <0.001 |
| 12-13 | 39 | 173.0±7.6 | 151.8±7.6 | <0.001 | 43 | 55.0±12.0 | 45.5±8.7 | <0.001 |
| 13-14 | 50 | 178.8±9.7 | 159.0±7.9 | <0.001 | 51 | 57.4±13.8 | 50.7±9.3 | 0.001 |
| 14-15 | 34 | 184.8±7.7 | 165.5±7.4 | <0.001 | 44 | 66.1±13.1 | 55.4±9.5 | <0.001 |
| 15-16 | 30 | 186.9±8.4 | 169.7±6.5 | <0.001 | 34 | 66.3±13.8 | 59.4±9.3 | 0.006 |
| 16-17 | 29 | 187.1±9.5 | 171.8±5.7 | <0.001 | 33 | 69.4±12.6 | 62.4±8.8 | 0.003 |
| 17-18 | 20 | 189.7±9.0 | 172.8±5.5 | <0.001 | 19 | 74.0±11.8 | 64.5±8.3 | 0.002 |
| 18-19 | 17 | 189.8±4.4 | 173.4±5.6 | <0.001 | 24 | 71.5±11.3 | 65.8±8.2 | 0.02 |
| 19-20 | 17 | 191.5±5.3 | 23 | 75.3±8.4 |
MFS, Marfan syndrome; SD, Standard deviation; No., Numbers of measurement; Ht, Height; Wt, Weight.
Table 2. Anthropometric data of females with Marfan syndrome compared to the general population.
| Age (yr) | No. (Ht) | Mean Height±SD of MFS (cm) | Mean Height±SD of Normal (cm) | P | No. (Wt) | Mean Weight±SD of MFS (kg) | Mean Weight±SD of Normal (kg) | P |
|---|---|---|---|---|---|---|---|---|
| 0-1 | 10 | 65.6±9.5 | 66.3±2.7 | 0.832 | 14 | 5.5±2.3 | 7.5±0.8 | 0.022 |
| 1-2 | 10 | 90.4±4.8 | 80.8±3.2 | <0.001 | 11 | 12.0±1.7 | 10.7±1.2 | 0.039 |
| 2-3 | 16 | 99.7±1.8 | 89.8±3.7 | <0.001 | 19 | 14.0±0.7 | 12.9±1.4 | 0.001 |
| 3-4 | 23 | 109.0±3.9 | 97.1±4.0 | <0.001 | 27 | 16.3±3.3 | 14.7±1.5 | 0.017 |
| 4-5 | 27 | 117.0±4.0 | 104.2±4.1 | <0.001 | 29 | 19.4±4.0 | 16.7±1.7 | 0.001 |
| 5-6 | 36 | 124.3±4.8 | 110.7±4.3 | <0.001 | 37 | 21.7±4.4 | 18.9±2.0 | <0.001 |
| 6-7 | 32 | 130.6±5.1 | 116.7±4.6 | <0.001 | 35 | 24.7±5.6 | 21.2±2.5 | 0.001 |
| 7-8 | 31 | 134.9±6.4 | 122.4±5.0 | <0.001 | 30 | 25.4±4.7 | 23.9±3.2 | 0.092 |
| 8-9 | 35 | 143.6±6.6 | 127.8±5.5 | <0.001 | 34 | 28.5±4.6 | 26.9±4.1 | 0.053 |
| 9-10 | 28 | 150.8±6.0 | 133.5±6.1 | <0.001 | 28 | 33.7±5.1 | 30.5±5.1 | 0.003 |
| 10-11 | 30 | 152.4±6.9 | 139.9±6.7 | <0.001 | 30 | 34.9±6.8 | 34.7±6.0 | 0.839 |
| 11-12 | 25 | 159.4±7.6 | 146.7±7.0 | <0.001 | 24 | 39.8±8.7 | 39.2±6.8 | 0.768 |
| 12-13 | 42 | 168.1±5.4 | 152.7±6.6 | <0.001 | 49 | 48.9±9.2 | 43.8±7.3 | <0.001 |
| 13-14 | 74 | 173.4±7.2 | 156.6±5.9 | <0.001 | 76 | 51.1±9.5 | 47.8±7.5 | 0.004 |
| 14-15 | 50 | 173.8±8.0 | 158.5±5.3 | <0.001 | 54 | 51.4±8.2 | 50.9±7.4 | 0.699 |
| 15-16 | 37 | 174.2±6.9 | 159.4±5.1 | <0.001 | 39 | 52.9±9.9 | 52.8±7.0 | 0.515 |
| 16-17 | 38 | 174.5±5.8 | 160.0±5.1 | <0.001 | 40 | 56.2±8.0 | 53.6±6.45 | 0.051 |
| 17-18 | 17 | 176.6±6.6 | 160.4±5.1 | <0.001 | 28 | 57.1±7.8 | 53.9±6.0 | 0.038 |
| 18-19 | 18 | 177.1±6.0 | 160.7±5.0 | <0.001 | 28 | 57.2±6.4 | 54.1±5.8 | 0.017 |
| 19-20 | 18 | 176.2±5.4 | 33 | 56.6±8.4 |
MFS, Marfan syndrome; SD, Standard deviation; No., Numbers of measurement; Ht, Height; Wt, Weight.
Fig. 1. Growth curve of patients with Marfan syndrome. (A) Male. (B) Female. The data of MFS are shown as solid lines and the data of general population as dotted lines on this growth chart.
With regard to the height of the female MFS, the 3rd percentile for MFS coincided with the 75th percentile of the standard curve by 13 yr of age, and remained between the 50th and 75th percentile by 15 yr (Fig. 1B). The mean MFS adult height was 177.1±6.0 cm, and it was above the 97th percentile for Korean adult females (170.7±5.3 cm, P<0.001).
Body weight growth curve
In males, except for two age groups, the MFS mean body weight was larger than that of the general population (Table 1). With regard the weight of the male MFS, the 50th percentile was between the 75th percentile and the 97th percentile of the standard curve by 5 yr of age and almost coincided with the 75th percentile of the standard curve by 8 yr of age (Fig. 1). The mean MFS adult body weight was 71.5±11.3 kg, which is larger than the mean body weight of Korean adult males (65.8±8.2 kg, P=0.02), but under the 97th percentile for Korean adult males (88.9±11.1 kg, P<0.001).
In females, there were significant differences in the mean body weight between MFS patients and the general population among most of the age groups (Table 2). However, in Fig. 1B, the 50th percentile of the MFS curve followed the 75th percentile of the standard curve by 8 yr of age (Fig. 1B) and it remains between the 50th percentile and the 75th percentile of standard curve. Then the 50th percentile of the MFS curve coincided with the 50th percentile of the standard curve (Fig. 1B). The mean MFS adult body weight was 57.2±6.4 kg, which was larger than the mean body weight of Korean adult females (54.1±5.8 kg, P=0.017), but under the 97th percentile for Korean adult females (71.2±7.5 kg, P<0.001).
Linear growth pattern
As there was no reference data of growth velocity for Korean children, we compared the linear growth pattern through analyzing longitudinal height parameters in the two groups. By 8.4 yr of age, the mean height of MFS males had reached 75 percent of the adult height. During the ten years from 4 to 14 yr of age, MFS males grew approximately 6.2 cm per year, and after 15 yr of age, their linear growth velocity was less than 2 cm per year. In the general population, by 8.7 yr of age, the mean height had reached 75 percent of the adult height. During the same period, normal children grew 5.3 cm per year, and after 15 yr of age, their annual linear growth velocity was less than 2 cm.
For females, by 7.0 yr of age, the mean height of MFS females had reached 75 percent of the adult height. During the ten years from 4 to 14 yr of age, MFS females grew 5.6 cm per year, and after 14 yr of age, their linear growth velocity was less than 2 cm per year. In the general population, by 7.4 yr of age, the mean height had reached 75 percent of the adult height. During the same period, normal children grew 5.2 cm per year, and after 14 yr of age, their annual linear growth velocity was less than 2 cm.
DISCUSSION
The disease-specific growth chart is an important clinical tool for monitoring the growth of patients with conditions that cause growth impairment, such as Down syndrome (13, 14, 15), Turner syndrome (16), and Prader-Willi syndrome (17). In the case of MFS, Erkula et al. (5) first reported the MFS-specific growth chart in the US. These charts have generally been used in clinical centers for monitoring the growth and the effect of hormonal therapy with these syndromes.
According to Erkula et al. (5), the mean height at birth in MFS patients coincided with the 90th percentile of the general population in both genders, which indicates that excessive linear growth in MFS patients begins prenatally. However in the current study, we could not analyze the statistical differences of birth height and weight between MFS and the general population because the data of birth length and birth weight in MFS patients were not sufficient for comparison. After 2 yr of age, the 50th percentile curve for Korean MFS linear growth was above the 97 percentile of the normal group in both genders. This was analogous to the American MFS height curve (5). Furthermore, the final MFS adult heights in Korea (191.4±5.2 cm for males and 176.2±5.3 cm for females) and the American (191.3±9.0 cm for males and 175.4±8.2 cm for females) were nearly equivalent, despite the difference in mean adult heights between the countries (173.3 cm vs. 176.7 cm for males, 160.7 cm vs. 163.1 cm for females). During the two years after birth, the MFS mean height was approximately 10 cm larger than that of the general population. However, in both gender, there was no significant difference in the age to reach the 75th percentile of the final adult height between MFS and general population. In males older than 15 yr old and females older than 14 yr old, the annual difference of mean height was less than 2 cm, so the timing of the cessation of meaningful linear growth was similar to both in general population and MFS. Therefore, our study supports the American data, in which the above average height in MFS patients was obtained through early rapid growth and a broader and higher growth velocity of preadolescent and adolescent periods (5). Regarding body mass, although the mean body weight of MFS patients was larger than that of the general population in males and females, the gap of the mean weight curve was small against the height curve, especially for females. These results may partially explain marfanoid features such as tallness and a slender figure. In reports about ethnic phenotype differences in Korean and Japanese MFS as compared to the Western MFS population, Korean and Japanese MFS patients had a lower frequency of skeletal manifestations such as arm span to height ratio, scoliosis, reduced extension at the elbows, and joint hypermobility (6, 7). However, in this study, the growth curve, pattern, and even final adult height were nearly analogous to those of America.
To our knowledge, this study constitutes the first growth chart data for Korean patients with MFS, and there was no disease-specific growth chart for Asian MFS patients before this study. However, the study has several limitations. First, the study was not performed among the nationwide institutions in Korea. Most of the MFS patients have been referred to Marfan clinic in our hospital when they are diagnosed MFS, because our institution has the biggest MFS clinic in Korea and the patients come from all around Korea. Therefore, it is regarded that our charts may represent the data of Korean MFS patients. Second, we did not have sufficient longitudinal data during infancy included in the birth data; because except for some patients who received an early genetic diagnosis due to family history of MFS or those with a severe phenotype, the diagnosis of infant MFS is very difficult. Therefore, our longitudinal growth data of the period during first 24 months, when height and body mass growth were the most active, was relatively insufficient compared with that of the subsequent period. Third, we had difficulty interpreting the growth pattern due to the lack of reference data about growth velocity in Korean children, so we could not account for the difference of the prepubertal and pubertal growth peak between MFS patients and the general population. Finally, we could not obtain radiologic findings for the identification of skeletal maturity, such as bone age, because the study was of a retrospective design. Therefore, further study is needed on multicenter based, with a prospective design, including sufficient anthropometric data on the birth and infant period and the bone age data.
Despite these limitations, we developed the first disease-specific growth charts for Korean MFS. We believe that the charts can be applied to those with MFS and to those treated with sex steroid hormone for growth reductive therapy.
Footnotes
This study was supported by Samsung Medical Center grant (#GFO 1140061) and Samsung Biomedical Research Institute grant (#SMO 1131471).
DISCLOSURE: All authors report no conflicts of interests and have nothing to disclose.
AUTHOR CONTRIBUTION: Conceived and designed the experiments: Kwun YH, Kim SJ, Jin DK. Performed the experiments: Kwun YH, Lee JE, Isojima T. Analyzed the data: Kwun YH, Kim SJ, isojima T, Cho SY, Jin DK. Kim TH, Kim JE. Contributed reagents/materials/analysis tools: Kwun YH, Kim SJ, Cho SY. Wrote the first draft of the manuscript: Kwun YH, Kim SJ. Wrote the paper: Kwun YH, Kim SJ, Cho SY, Jin DK. Agree with conclusions and final manuscript: all authors.
References
- 1.Pyeritz RE, McKusick VA. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979;300:772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- 2.Lee NC, Hwang B, Chen CH, Niu DM. Intrafamilial phenotype variation in Marfan syndrome ascertained by intragenic linkage analysis. J Formos Med Assoc. 2005;104:964–967. [PubMed] [Google Scholar]
- 3.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray JR, Bridges AB, West RR, McLeish L, Stuart AG, Dean JC, Porteous ME, Boxer M, Davies SJ. Life expectancy in British Marfan syndrome populations. Clin Genet. 1998;54:124–128. doi: 10.1111/j.1399-0004.1998.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 5.Erkula G, Jones KB, Sponseller PD, Dietz HC, Pyeritz RE. Growth and maturation in Marfan syndrome. Am J Med Genet. 2002;109:100–115. doi: 10.1002/ajmg.10312. [DOI] [PubMed] [Google Scholar]
- 6.Akutsu K, Morisaki H, Takeshita S, Ogino H, Higashi M, Okajima T, Yoshimuta T, Tsutsumi Y, Nonogi H, Morisaki T. Characteristics in phenotypic manifestations of genetically proved Marfan syndrome in a Japanese population. Am J Cardiol. 2009;103:1146–1148. doi: 10.1016/j.amjcard.2008.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Yoo EH, Woo H, Ki CS, Lee HJ, Kim DK, Kang IS, Park P, Sung K, Lee CS, Chung TY, et al. Clinical and genetic analysis of Korean patients with Marfan syndrome: possible ethnic differences in clinical manifestation. Clin Genet. 2010;77:177–182. doi: 10.1111/j.1399-0004.2009.01287.x. [DOI] [PubMed] [Google Scholar]
- 8.Yang JH, Han H, Jang SY, Moon JR, Sung K, Chung TY, Lee HJ, Ki CS, Kim DK. A comparison of the Ghent and revised Ghent nosologies for the diagnosis of Marfan syndrome in an adult Korean population. Am J Med Genet A. 2012;158a:989–995. doi: 10.1002/ajmg.a.34392. [DOI] [PubMed] [Google Scholar]
- 9.Aragon-Martin JA, Ahnood D, Charteris DG, Saggar A, Nischal KK, Comeglio P, Chandra A, Child AH, Arno G. Role of ADAMTSL4 mutations in FBN1 mutation-negative ectopia lentis patients. Hum Mutat. 2010;31:E1622–E1631. doi: 10.1002/humu.21305. [DOI] [PubMed] [Google Scholar]
- 10.Cole TJ, Green PJ. Smoothing reference centile curves: the LMS method and penalized likelihood. Stat Med. 1992;11:1305–1319. doi: 10.1002/sim.4780111005. [DOI] [PubMed] [Google Scholar]
- 11.Pan H, Cole TJ. LMSchartmaker, a program to construct growth references using the LMS method. Version 2.54. 2011. [accessed on 14 June 2015]. Available at http://www.healthforallchildren.com/?product=lmschartmaker-light.
- 12.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, Oh K, Jang MJ, Hwang SS, Yoo MH, et al. 2007 Korean National Growth Charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 13.Bertapelli F, Martin JE, Gonçalves EM, de Oliveira Barbeta VJ, Guerra-Júnior G. Growth curves in Down syndrome: implications for clinical practice. Am J Med Genet A. 2014;164a:844–847. doi: 10.1002/ajmg.a.36337. [DOI] [PubMed] [Google Scholar]
- 14.Su X, Lau JT, Yu CM, Chow CB, Lee LP, But BW, Yam WK, Tse PW, Fung EL, Choi KC. Growth charts for Chinese Down syndrome children from birth to 14 years. Arch Dis Child. 2014;99:824–829. doi: 10.1136/archdischild-2013-304494. [DOI] [PubMed] [Google Scholar]
- 15.Tüysüz B, Göknar NT, Oztürk B. Growth charts of Turkish children with Down syndrome. Am J Med Genet A. 2012;158a:2656–2664. doi: 10.1002/ajmg.a.35710. [DOI] [PubMed] [Google Scholar]
- 16.Isojima T, Yokoya S, Ito J, Horikawa R, Tanaka T. New reference growth charts for Japanese girls with Turner syndrome. Pediatr Int. 2009;51:709–714. doi: 10.1111/j.1442-200X.2009.02838.x. [DOI] [PubMed] [Google Scholar]
- 17.Butler MG, Sturich J, Lee J, Myers SE, Whitman BY, Gold JA, Kimonis V, Scheimann A, Terrazas N, Driscoll DJ. Growth standards of infants with Prader-Willi syndrome. Pediatrics. 2011;127:687–695. doi: 10.1542/peds.2010-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]