Abstract
Background
The purpose of this study was to evaluate the effect of implantable cardioverter defibrillators (ICD) in heart failure (HF) patients compared to pharmacologic/conventional management.
Material/Methods
We searched PubMed, Embase, and Springer Link Library databases up to February 10th, 2014. Pooled risk ratio (RR) and 95% confidence interval (CI) for the mortality of the patients with HF were collected and calculated in a fixed-effects model or a random-effects model, as appropriate. Summary effect estimates were also stratified by sex and follow-up time. Egger’s regression asymmetry tests were utilized for publication bias detection.
Results
A total of 7 separate studies including 15 520 patients (10 801 ICD cases and 4719 controls) with HF were considered in the meta-analysis. The overall estimates showed that ICD could statistically significantly reduce the mortality of male (RR=0.73, 95% CI: 0.66–0.80) and female (RR=0.75, 95% CI: 0.63–0.90) patients. In addition, the further stratification subgroup analysis indicated that ICD presented a significant reduction (male: RR=0.72, 95% CI: 0.64–0.81; female: RR=0.69, 95% CI: 0.56–0.85) of mortality after 2–3 years of ICD therapy. The RR (95% CI) effects of mortality after 4–5 years of ICD therapy for males and females were 0.76 (0.51–1.14) and 0.96 (0.68–1.37), respectively.
Conclusions
This meta-analysis suggests that ICD could reduce HF patient mortality despite the sex difference.
MeSH Keywords: Defibrillators, Implantable; Heart Failure; Meta-Analysis
Background
Heart failure (HF), as primarily a disease of the elderly, is a major public health problem in the United States, and the annual incidence of heart failure is approximately 10 per 1000 patients 65 years and older [1–4]. Moreover, nearly 300 000 patients die due to HF as a primary or contributory cause each year, and the number of deaths has increased steadily despite advances in treatment [2]. Despite enormous progress in treatment, HF mortality rates remain unacceptably high, with approximately 70–80% of patients dying within 8 years of diagnosis [3]. In patients diagnosed as having HF, sudden cardiac death (SCD) occurs at 6–9 times the rate of the general population [5], and it accounts for two-thirds of the sudden death cases in the United states [6]. A previous report also suggested that SCD occurred at a rate of 41.8/100 000 population in China, accounting for over 544 000 deaths annually [7]. Therefore, HF is associated with high mortality and morbidity.
The mechanism of sudden death is arrhythmic with about 75% of mortalities involving ventricular fibrillation or tachycardia [8]. The implantable cardioverter defibrillator (ICD), a battery-powered implantable device that can detect and terminate potentially life-threatening tachyarrhythmias via defibrillation to prevent SCD, has been continuously improved [9]. In addition, studies have demonstrated that ICD therapy could (significantly) reduce by 31% the risk of mortality in HF patients [10,11]. However, on the basis of a non-statistically significant finding, 2 previous reviews have shown there is little or no benefit from use of ICDs in women [12,13]. Hohnloser et al. reported that ICD therapy did not reduce overall mortality in high-risk patients who had recently had a myocardial infarction [14]. Thus, it is controversial whether the effect of ICD treatment is beneficial for HF patients. In the present work, we conducted a systematic meta-analysis to achieve an integrative understanding of the relative effects of ICD and pharmacologic therapy in HF patients.
Material and Methods
Source of material
We performed the pre-established search strategies and systematically retrieved studies from PubMed, Embase, and Springer Link databases until Feb. 10th, 2014. The key words of “defibrillator”, “implantable cardioverter defibrillators”, “heart failure”, “failure”, “mortality”, and “death” were used for all searches. A manual search of print documents and citations from relevant original studies and review articles performed for any additional studies. We only collected data from fully-published English papers, excluding any meeting or conference abstracts.
Study selection criteria
Inclusion criteria were: 1) research design was prospective cohort study or randomized controlled trial; 2) study objects were HF patients; 3) the experimental group was HF patients treated with ICD, and the control group was HF patients accepted with pharmacologic therapy or conventional therapy; and 4) the outcome of the included studies was the mortality of the HF patients with different treatment. Exclusion criteria were: 1) HF patients in experimental group received ICD treatment and also accepted other interventional therapy; 2) article was non-original literature such as review, letters, and comments; and 3) duplicate publications that used the same population data – only the one with longest follow-up and most complete information was included, and the rest were excluded.
Data extraction
Two reviewers independently extracted data from the primary studies into an Excel spreadsheet using a standardized form to assess the eligibility for inclusion. In brief, information were tabulated according to the article’s first author’s name, year of publication, region where research was conducted, sample size of the experimental group and control group, duration of follow-up, age and sex of the study individuals, and the adjusted RR and 95% CI. When completed, the information tables were exchanged and checked. Any discrepancies were resolved by discussion and by referring to the original publication.
Meta-analysis methods
In the present meta-analysis, the point estimates of RRs and its 95% CI were calculated as effect sizes. We assessed the heterogeneity by testing Cochran’s Q-statistic [15]. The effect of heterogeneity was also quantified using I2=100%×(Q−df)/Q[16]. If a significant Q-statistic (P<0.05) or I2-statistic (I2>50%) indicated heterogeneity across studies, then the random-effects model was used for meta-analysis. Otherwise, heterogeneity was not significant and the fixed-effects model was applied. The overall estimates of RRs was obtained using Mantel-Haenszel method in the fixed-effects model [17]or DerSimonian and Laid method in the random-effects model [18]. To test the reliability of the results, we also performed a sensitivity analysis by repeating the meta-analysis after removal of 1 study each time. Publication bias was assessed by Egger’s regression asymmetry test and P>0.05 for both tests was considered to be no significant publication bias [19].
Results
Characteristics of eligible studies
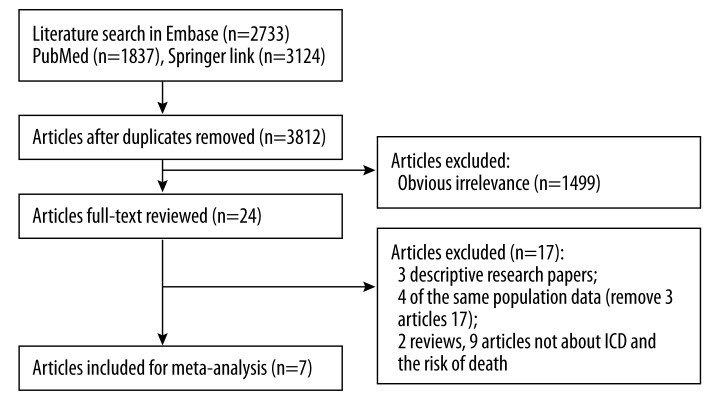
The details of the study selection process are shown in Figure 1. According to the pre-established search strategies, we found 7694 papers potentially relevant to the search terms (PubMed: 1837; Embase: 2733; Springer: 3124). A total of 3812 potentially relevant studies were selected after duplicates were removed. During the step of title and abstract screening, 3788 articles were excluded. Among the remaining 24 studies, only 7 (3 descriptive research papers, 4 with the same population data, 2 reviews, and 9 articles not about ICD and the risk of death) met the inclusion criteria after full publication review.
Figure 1.
Literature search and study selection.
Table 1 shows the characteristics of the 7 included studies [14,20–25], which were published between 2004 and 2014. A total of 15 520 patients (10 801 ICD cases and 4719 controls) with HF were considered in the meta-analysis. Sample sizes ranged from 34 to 2012, and average age was 57.9–75.3 years. Follow-up times were between 20 and 48 months.
Table 1.
Characteristics of studies included in the meta-analysis.
| ID | Author year | Country | Type of control | Follow up times | Years of event rates | Follow-up (%) | Mean LVEF | Sex | Control n, age | Case n, age | RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hohnloser 2004 | Germany | Pharmacologic therapy | 30 months* | 4 years | 100.00% | 0.28 | F | 80, 62.1 (10.6) | 80, 61.5 (10.9) | 1.00 (0.49, 2.04) |
| M | 252, 62.1 (10.6) | 262, 61.5 (10.9) | 1.14 (0.77, 1.69) | ||||||||
| 2 | Wilcox 2014 | USA | Pharmacologic therapy | 24 months# | 2 years | 99.96% | 0.25 | F | 1082, 69.3 (13.8) | 604, 69.3 (13.8) | 0.73 (0.55, 0.98) |
| M | 1985, 68.7 (12.8) | 1955, 68.7 (12.8) | 0.73 (0.57, 0.92) | ||||||||
| 3 | Hernandez 2010 | USA | Pharmacologic therapy | 48 months# | 3 years | 100.00% | ≤0.35 | F | 1797, 75.3 (5.6) | 99, 74.3 (5.5) | 0.58 (0.41, 0.83) |
| M | 2012, 75.3 (5.6) | 277, 74.3 (5.5) | 0.80 (0.63, 1.01) | ||||||||
| 4 | Russo 2008 | USA | Pharmacologic therapy | 45.5 months* | 5 years | 100.00% | 0.25 | F | 588, 60 (50, 67) | 0.90 (0.56, 1.43) | |
| 0.24 | M | 1933, 60 (52, 69) | 0.71 (0.57, 0.88) | ||||||||
| 5 | Zareba 2005 | USA | Conventional therapy | 20 months* | 2 years | 99.80% | ≤0.30 | F | 73, 64 (11) | 119, 64 (11) | 0.57 (0.28, 1.18) |
| M | 417, 65 (10) | 623, 65 (10) | 0.66 (0.48, 0.91) | ||||||||
| 6 | Russo 2004 | USA | Pharmacologic therapy | 39 months* | 2 years | 99.40% | ≤0.40 | F | 34, NP | 34, NP | 1.38 (0.63, 2.99) |
| M | 319, NP | 317, NP | 0.72 (0.55, 0.95) | ||||||||
| 7 | Albert 2008 | USA | Pharmacologic therapy | 29 months* | 5 years | 100.00% | 0.22 | F | 69, 59.1 | 63, 59.1 | 1.14 (0.50, 2.64) |
| 0.21 | M | 160, 57.9 | 166, 57.9 | 0.49 (0.27, 0.90) | |||||||
LVEF – left ventricular ejection fraction;
mean;
length;
F – female; M – male; NP – not provided.
Overall effects of mortality and subgroup analysis
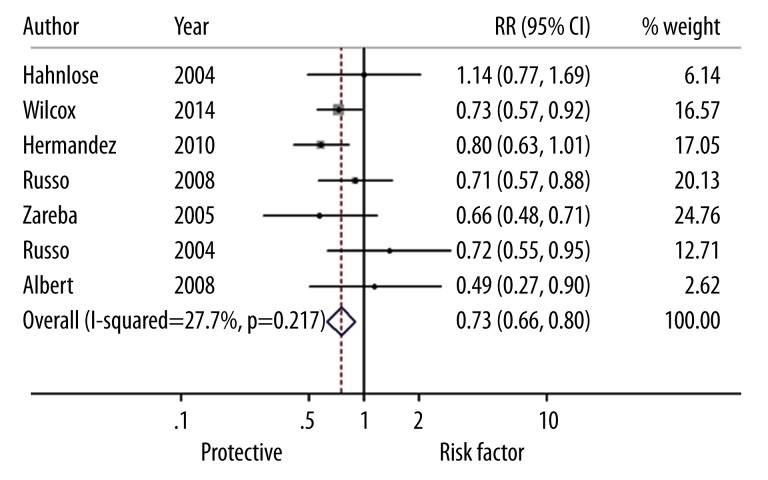
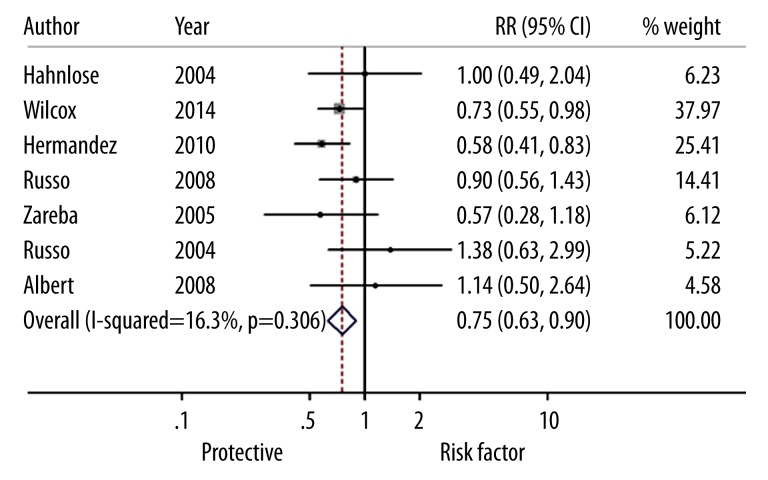
The summary of the meta-analysis for mortality of male patients with HF is shown in Figure 2. The heterogeneity test showed that there were no significant heterogeneities between studies (I2=27.7%, P=0.217), so we used the fixed-effects model to calculated the effect sizes of mortality of ICD cases vs. the controls. The overall estimates of mortality (RR=0.73, 95% CI: 0.66–0.80) indicated that ICD significantly decreased the death of male patients with HF compared to the pharmacologic therapy or conventional therapy. There was no heterogeneity between studies (I2=16.3%, P=0.306) for the female patients. The fixed-effects model, showed the pooled estimate of RR was 0.75 (95% CI: 0.63–0.90), which demonstrates that ICD could significantly reduce the mortality of female patients with HF (Figure 3).
Figure 2.
Forest plots for risk ratios of mortality (male) in heart failure patients associated with the implantation of cardioverter defibrillator treatment vs. pharmacologic therapy or conventional therapy. Squares represent the effect size for the risk ratio of mortality in heart failure patients associated with the cardioverter defibrillator treatment vs. the pharmacologic therapy or conventional therapy. Size of the squares is proportional to the size of the cohorts. Error bars represent 95% confidence intervals (CI). The diamond shape represents the pooled estimates within each analysis.
Figure 3.
Forest plots for risk ratios of mortality (female) in heart failure patients associated with the implantation of cardioverter defibrillator treatment vs. pharmacologic therapy or conventional therapy. Squares represent the effect size for the risk ratio of mortality in heart failure patients associated with the cardioverter defibrillator treatment vs. pharmacologic therapy or conventional therapy. Size of squares is proportional to the size of the cohorts. Error bars represent 95% confidence intervals (CI). The diamond shape represents the pooled estimates within each analysis.
Subgroup analysis stratified by the time after receiving an ICD was performed and results are shown in Table 2. The result of reduced mortality in HF patients was consistently found in the stratified analyses 2–3 years after receiving an ICD. But in the 4–5 years subgroup analysis, the effect sizes of male (RR=0.76, 95% CI: 0.51–1.14, P=0.184) and female patients (RR=0.96, 95% CI: 0.68–1.37, P=0.840) demonstrated that ICD use did not significantly reduce the death rate.
Table 2.
Pooled risk ratios of mortality for ICD treatment cases vs. control group cases in the meta-analysis.
| Total/subgroup | Category | No. of studies | RR (95% CI) | Heterogeneity test | |
|---|---|---|---|---|---|
| P | I2 (%) | ||||
| Male | |||||
| All studies | 7 | 0.73 (0.66, 0.80) | 0.217 | 27.7 | |
| Years of event rates | 2–3 years | 4 | 0.72 (0.64, 0.81) | 0.674 | 0.0 |
| 4–5 years | 3 | 0.76 (0.51, 1.14) | 0.039 | 69.3 | |
| Female | |||||
| All studies | 7 | 0.75 (0.63, 0.90) | 0.306 | 16.3 | |
| Years of event rates | 2–3 years | 4 | 0.69 (0.56, 0.85) | 0.222 | 31.7 |
| 4–5 years | 3 | 0.96 (0.68, 1.37) | 0.883 | 0.0 | |
Sensitivity analysis and publication bias
The outcomes of RR in the sensitivity analysis of male patients ranged from 0.71 (95% CI: 0.64–0.78) to 0.75 (95% CI: 0.66–0.82) and the RR in the female patients ranged from 0.73 (95% CI: 0.60–0.88) to 0.25 (95% CI: 0.69–1.00). Therefore, the results of this meta-analysis are statistically stable and reliable.
The Egger’s linear regression test was used to assess publication bias. For the male and female patent studies, the P values of mortality were 0.760 and 0.155, respectively, which revealed no statistically significant publication bias.
Discussion
Many studies have compared ICD with pharmacologic therapy or conventional therapy in HF patients, but these studies have shown mixed results due to small sample sizes, low statistical power, patient sex, and different regions. In this meta-analysis, we combined and re-analyzed 7 studies including 15 520 patients (10 801 ICD cases and 4719 controls) assess the effectiveness of ICD for treatment of HF.
The results of this meta-analysis showed that HF significantly reduced the mortality of female patients (RR=0.75, 95% CI=0.63–0.90) and male patients (RR=0.73, 95% CI=0.66–0.80) vs. pharmacologic/conventional management, consistent with a previous study reporting that the RR (95% CI) of female and male mortality in Wilcox’s paper were 0.73 (0.55–0.8) and 0.73 (0.57–0.9), respectively. In addition, the mortality benefit of ICD use was also reported in Russo’s studies [22,25], despite the small number of women enrolled, and did not appear to be influenced by sex in these previous trials. Our subgroup analysis also demonstrated that ICD use could significantly reduce the mortality in HF patients without differences according to sex, after receiving the therapy for 2–3 years;, and even after 4–5 years the mortality was still less than the pharmacologic/conventional treatment, but this result was not significant, perhaps because of the relatively small population included.
ICD therapy was intentionally selected to consist of shock-only, single-lead therapy with the goal of treating only rapid, sustained ventricular tachycardia or ventricular fibrillation [10]. In addition, the ICD was uniformly programmed to have a detection rate of 187 beats per minute or more [26]. Anti-tachycardia pacing therapies were not permitted in order to minimize excessively rapid intervention in the event of non-sustained ventricular tachycardia [10,26]. In recent years, ICD therapy has been shown to be effective in the reduction of mortality of HF patient in numerous studies [11,22,25]. Although Kadish showed no reduction in all-cause mortality for female HF patents receiving ICD therapy [27], our analysis indicate that ICD could be effectively used for all eligible patients without sex difference.
Since the first descriptions of external defibrillation in the 1960s and the first human ICD application in 1980 by Mirowski [28], the paradigm for the prevention of sudden cardiac death has shifted away from anti-arrhythmic drug and ablative strategies. In the past 15 years, the annual insertion of ICDs has increased by 20-fold in the United States [29]. Several specific cardiomyopathies and hereditary heart diseases are endemic in China and about 70% of SCD are due to these heart diseases, but the main strategy for SCD is pharmacological treatment (with high mortality), as ICD therapy is limited by socioeconomic conditions [7]. Inspiringly, there is now increasing use of ICD in China and a national project has been recently started for SCD prevention.
The present study was an updated meta-analysis of the effects of ICD therapy on mortality in adults with HF. However, some limitations of this study should be noted. Firstly, few articles were included, and were mainly conducted in the United States, so the outcomes might not be generalizable to patients in others countries. Therefore, it is necessary to conduct investigations in Asian, Australian, Central and South American, and European countries. Secondly, age, health status, quality of life, and patient condition were not uniform, so the results should be interpreted with caution. Thirdly, we did not have information on the pharmacologic/conventional treatment, such as medications intake and doses, and we did not account for the small number of patients who crossed over from the comparison group to the ICD group after the index hospitalization. Finally, we did not consider the complications of ICD treatment, which is important in evaluating the use of ICD therapy.
Conclusions
Patients with HF had significantly less mortality when treated with ICD compared to pharmacologic/conventional management.
Acknowledgments
We would like to thank all respondents of the study and all the people who helped us in this study.
Footnotes
Conflict of interest statement
The authors have declared that no competing interests exist.
Source of support: Self financing
References
- 1.Kannel WB, Belanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–57. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Baker DW, Chin MH, et al. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summaryA report of the american college of cardiology/american heart association task force on practice guidelines (committee to revise the 1995 guidelines for the evaluation and management of heart failure) developed in collaboration with the international society for heart and lung transplantation endorsed by the heart failure society of america. J Am Coll Cardiol. 2001;38:2101–13. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, Larson MG, Leip EP, et al. Lifetime risk for developing congestive heart failure the Framingham Heart Study. Circulation. 2002;106:3068–72. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.Said S, Cooper CJ, Alkhateeb H, et al. Incidence of new onset atrial fibrillation in patients with permanent pacemakers and the relation to the pacing mode. Med Sci Monit. 2014;20:268–73. doi: 10.12659/MSM.890052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Heart Association. Heart disease and stroke statistics: 2008 update at-a-glance. 2008. Retrieved October 2008, 31. [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) State-specific mortality from sudden cardiac death – United States, 1999. Morb Mortal Wkly Rep. 2002;51:123–26. [PubMed] [Google Scholar]
- 7.Zhang S. Sudden cardiac death in China. Pacing Clin Electrophysiol. 2009;32:1159–62. doi: 10.1111/j.1540-8159.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 8.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 9.Glikson M, Friedman PA. The implantable cardioverter defibrillator. Lancet. 2001;357:1107–17. doi: 10.1016/S0140-6736(00)04263-X. [DOI] [PubMed] [Google Scholar]
- 10.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz JA, Rowe BH, Dryden DM, et al. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med. 2007;147:251–62. doi: 10.7326/0003-4819-147-4-200708210-00007. [DOI] [PubMed] [Google Scholar]
- 12.Ghanbari H, Dalloul G, Hasan R, et al. Effectiveness of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in women with advanced heart failure: a meta-analysis of randomized controlled trials. Arch Intern Med. 2009;169:1500–6. doi: 10.1001/archinternmed.2009.255. [DOI] [PubMed] [Google Scholar]
- 13.Santangeli P, Pelargonio G, Russo AD, et al. Gender differences in clinical outcome and primary prevention defibrillator benefit in patients with severe left ventricular dysfunction: a systematic review and meta-analysis. Heart Rhythm. 2010;7:876–82. doi: 10.1016/j.hrthm.2010.03.042. [DOI] [PubMed] [Google Scholar]
- 14.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351:2481–88. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 15.Deeks JJ, Altman DG, Bradburn MJ. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. 2001. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis; pp. 285–312. [Google Scholar]
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. The Challenge of Epidemiology: Issues and Selected Readings. 2004;1:533–53. [PubMed] [Google Scholar]
- 18.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 19.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–101. [PubMed] [Google Scholar]
- 20.Wilcox JE, Fonarow GC, Zhang Y, et al. Clinical effectiveness of cardiac resynchronization and implantable cardioverter-defibrillator therapy in men and women with heart failure findings from IMPROVE HF. Circ Heart Fail. 2014;7:146–53. doi: 10.1161/CIRCHEARTFAILURE.113.000789. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez AF, Fonarow GC, Hammill BG, et al. Clinical effectiveness of implantable cardioverter-defibrillators among Medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3:7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]
- 22.Russo AM, Poole JE, Mark DB, et al. Primary prevention with defibrillator therapy in women: results from the Sudden Cardiac Death in Heart Failure Trial. J Cardiovasc Electrophysiol. 2008;19:720–24. doi: 10.1111/j.1540-8167.2008.01129.x. [DOI] [PubMed] [Google Scholar]
- 23.Albert CM, Quigg R, Saba S, et al. Sex differences in outcome after implantable cardioverter defibrillator implantation in nonischemic cardiomyopathy. Am Heart J. 2008;156:367–72. doi: 10.1016/j.ahj.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 24.Zareba W, Moss AJ, Jackson Hall W, et al. Clinical course and implantable cardioverter defibrillator therapy in postinfarction women with severe left ventricular dysfunction. J Cardiovasc Electrophysiol. 2005;16:1265–70. doi: 10.1111/j.1540-8167.2005.00224.x. [DOI] [PubMed] [Google Scholar]
- 25.Russo AM, Stamato NJ, Lehmann MH, et al. Influence of gender on arrhythmia characteristics and outcome in the Multicenter UnSustained Tachycardia Trial. J Cardiovasc Electrophysiol. 2004;15:993–98. doi: 10.1046/j.1540-8167.2004.04050.x. [DOI] [PubMed] [Google Scholar]
- 26.Streitner F, Kuschyk J, Dietrich C, et al. Comparison of ventricular tachyarrhythmia characteristics in patients with idiopathic dilated or ischemic cardiomyopathy and defibrillators implanted for primary prevention. Clin Cardiol. 2011;34:604–9. doi: 10.1002/clc.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151–58. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 28.Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. New Engl J Med. 1980;303:322–24. doi: 10.1056/NEJM198008073030607. [DOI] [PubMed] [Google Scholar]
- 29.Maisel WH, Moynahan M, Zuckerman BD, et al. Pacemaker and ICD generator malfunctions: analysis of Food and Drug Administration annual reports. JAMA. 2006;295:1901–6. doi: 10.1001/jama.295.16.1901. [DOI] [PubMed] [Google Scholar]