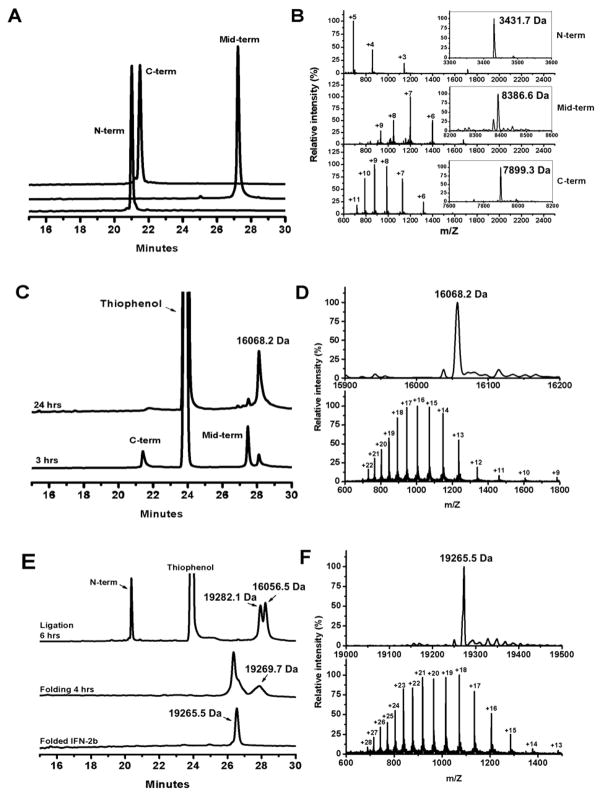
Fig 2.
Native chemical ligation reactions monitored by analytical RP-HPLC and ESI-MS. All HPLC chromatograms were obtained at 40 °C on a Waters Symmetry 300 C18 column (4.6×150 mm, 5 μm) running a linear gradient of 5% - 65 % of acetonitrile containing 0.1% TFA at a flow rate of 1 ml/min over 30 min. (A) HPLC traces of the three synthetic peptide fragments, Thz-Cys(2–28)IFNα-COSR, Thz-Cys(30–97)IFNα-COSR and (98–165)IFNα, and their ESI-MS data (B). (C) The first ligation reaction monitored by HPLC and verified by ESI-MS (D). (E) The second ligation reaction and protein oxidative folding monitored by HPLC and verified by ESI-MS (F). As expected, protein folding shortens retention time of IFNα2b as its hydrophobic residues are buried in the folded structure. The determined molecular mass of folded synthetic IFNα2b of 19265.5 Da is within experimental error of the theoretical value of 19265.1 Da calculated on the basis of the average isotopic compositions of IFNα2b.