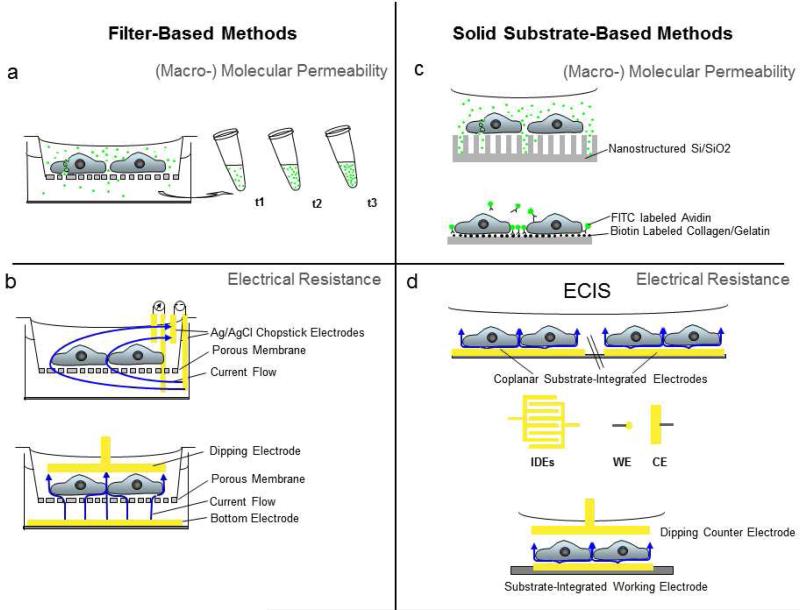
Fig. 2.
Overview of popular in vitro systems for study of barrier function using cultured endothelial cells.a, b Filter-based methods: Cultured endothelial cells are grown onto permeable filter supports to measure a passage of tracer molecules across an endothelial cell layer or b transendothelial electrical resistance (TEER) using a 4-electrode (upper panel) or 2-electrode setup (lower panel). Yellow bars indicate electrodes of conductive material (e.g. Ag, Au, stainless steel, platinum, indium tin oxide). Blue arrows indicate current flow, which is unidirectional for DC or bidirectional for AC signals (drawn unidirectionally for clarity). c, d Solid substrate based methods: c Recently developed techniques for molecular permeability using macroporous silicon that serve as microcuvettes (upper panel) or specific biotin/avidin interactions to immobilize tracer molecules at the site of molecule passage through the cell layer (lower panel). d Cells directly grown onto substrate-integrated electrodes are the basis for ECIS and related electrical techniques. Typically, substrate-integrated electrodes are in coplanar arrangement (upper setup). Note that the counter electrode (CE) is usually significantly larger than the working electrode (WE). In this setup the ECIS signal is dominated by changes that occur at the small working electrode. With the use of interdigitated electrodes (IDEs) with finger-like patterns, both electrodes contribute equally to the overall impedance signal. Alternatively, the counter electrode can be submersed into the culture medium approaching from the top (lower setup). Only the working electrode is substrate integrated. In this case the counter dipping electrode needs to have an at least 500-fold bigger surface area to make its contribution to the measurement negligible.