Abstract
Background
Interleukin-6 (IL-6), as one of the most typical pro-inflammatory and immunoregulatory cytokines, is believed to be associated with the genesis and maintenance of inflammatory response. Genetic association studies (GAS) that have investigated the association between Interleukin 6 (G174C and G572C) promoter gene polymorphisms and susceptibility to ischemic stroke (IS) which have produced contradictory and unconvincing results.
Purpose
The aim of this meta-analysis is to provide a relatively comprehensive account of the association of IL-6 (G174C and G572C) polymorphisms with susceptibility to IS.
Methods
A literature search was conducted using electronic database PubMed, Medline, and Trip database for all case-control studies investigating for association of IL-6 genetic polymorphisms with ischemic stroke published till August 30, 2014. The following combinations of main keywords were used: (‘Interleukin-6’ or ‘IL-6’) and (‘ischaemic stroke or ‘cerebral infarction’ or ‘IS’) and (‘genetic polymorphism’ or ‘single nucleotide polymorphisms’ or ‘SNP’). Pooled Odds ratios (ORs) and 95% confidence intervals (CIs) were determined for IL-6 gene-disease association. Meta-analysis was carried out using Revman 5.3 software.
Results
16 case-control studies involving a total of 3,317 IS patients and 3,432 healthy controls for G174C polymorphism and 3 case-control studies with a total of 2,001 IS patients and 2,027 healthy controls for G572C IL-6 gene polymorphisms were included in a meta-analysis. For IL-6 G174C gene polymorphisms, no significant association was observed under dominant [GC + CC vs. GG: OR = 1.01, 95% CI: 0.77–1.34, P = 0. 92], recessive [CC vs. GG + GC: OR = 0.82, 95% CI: 0.40–1.70, P = 0. 59] and allelic model [C vs. G Allele: OR = 0.99, 95% CI: 0.74–1.31, P = 0. 93]. For IL-6 G572C, no significant association was observed under dominant [CC vs. GG + GC: OR = 0.99, 95% CI: 0.57–1.71, P = 0. 97], recessive [CC vs. GG + GC: OR = 0.93, 95% CI: 0.60–1.45, P = 0. 75] and allelic model [C vs. G Allele: OR = 0.95, 95% CI: 0.66–1.36, P = 0. 76].
Conclusion
This meta-analysis shows that IL-6 (G174C) and IL-6 (G572C) gene polymorphisms may not be associated with an increased susceptibility to IS. Further studies are required for confirmatory results.
Keywords: Ischaemic stroke, Inflammatory gene, Single nucleotide Polymorphisms, Interleukin-6, Cytokine, Meta-analysis
 Introduction
Introduction
Stroke is the third leading cause of death worldwide after Ischemic Heart Disease (IHD) and Cancer. Stroke has accounted for nearly 5.7 million deaths worldwide, 87% of these deaths occur in low and middle income countries.1 Incidence of stroke in South Asian countries has increased by more than 100%, while this is decreased by 42% in developed European countries in last four decades.2 Ischaemic stroke (IS) accounts for 85% of stroke and its pathophysiology are regulated by a combination of lifestyle, environmental and unclear genetic risk factors.3 Inflammation and genetics are both prominent mechanisms in the pathogenesis of ischemic stroke.4 Candidate genes, stroke susceptibility alleles and their association with stroke pathogenesis have been intensely studied in the last few years.5,6
Human Interleukin-6 (IL-6) gene is located at chromosome 7p21 which consists of 5 exons and 4 introns and synthesized as a precursor protein of 232 amino acids.7,8 IL-6 is a pleiotropic cytokine associated with atherosclerosis and cardiovascular disease that may also be a key mediator in the inflammatory response to ischemic stroke.9 Two functional promoter polymorphisms, G174C and G572C have been identified in the IL-6 promoter region and these two genetic variants may be associated with the increased level of IL-6.10 The levels of IL-6 were found to be increased in both serum and cerebro-spinal fluid after ischemic stroke, and elevated IL-6 levels have been associated with greater stroke severity, larger final infarct volume, early neurological worsening, and worse functional outcome.11–18
Numerous studies have investigated the association of IL-6 G174C and G572C gene polymorphisms in relation to various ischaemic and atherosclerotic cardiovascular diseases. Associations have been reported between the GG genotype and asymptomatic carotid artery atherosclerosis,19–22 risk of coronary heart disease,23 peripheral arterial occlusive disease,24 multi-infarct dementia,25 and longer hospital and intensive care unit stay after coronary artery bypass graft surgery.26 However, other studies have found associations between the CC genotype and asymptomatic carotid artery atherosclerosis and increased mortality among abdominal aortic aneurysm patients.27 Reasons for these contradictory findings are unclear.
Inflammation and ischemic stroke are interrelated as many studies suggest that IL-6 may play a central role in the inflammatory response to cerebral ischemia.28–43 However, although the role of IL-6 in ischemic stroke has been extensively studied, the influence of IL-6 genetic polymorphisms on stroke is not well understood. The purpose of this meta-analysis is to summarize the studies that have examined the IL-6 (G174C and G572C) polymorphism in relation to ischemic stroke and to discuss the implications of these results for future research.
Methods
Identification of Relevant Studies
A literature search for GAS that investigated the association between the IL-6 gene polymorphisms and susceptibility to IS published before August 30, 2014 which was conducted in the following electronic databases: PubMed, Medline and trip databases. The following combinations of main keywords were used: (‘Interleukin-6’ or ‘IL-6’) and (‘ischaemic stroke or ‘cerebral infarction’ or ‘IS’) and (‘genetic polymorphism’ or ‘single nucleotide polymorphisms’ or ‘SNP’). The search was done without limitations on language, but only included those studies that were conducted on human subjects. All references in eligible articles were extensively reviewed to identify additional published articles.
Inclusion and Exclusion Criteria
To be included in the analysis, eligible studies had to meet the following criteria: (a) case-control studies on the association between the IL-6 gene polymorphisms and susceptibility to IS; (b) all patients in the candidate studies meet the diagnostic criteria for IS; (c) studies with sufficient available data to calculate Odds Ratios (ORs) with corresponding 95% Confidence intervals (CIs). The major reason for excluding studies were: (i) not case-control study; (ii) duplicates publications with overlapping subjects from the same study; and (iii) no available data reported. For multiple studies using overlapping cases or controls, the most recent study with the largest sample size was included in the meta-analysis. This metaanalysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines with only slight modification, and did not require ethics board approval.44
Data Extraction
According to the PRISMA guidelines, two investigators independently PK and AKY checked each full-text report for eligibility and extracted the following data from eligible studies: surname of first author, year of publication, country of origin, ethnicity, number of case and control, age, sex ratio, genotyping method, allele and genotype frequency etc. Disagreements were solved by discussion between all authors until consensus was reached.
Quality Assessment
Newcastle-Ottawa Scale (NOS) criteria45 were used to assess the qualities of all included studies. The NOS criteria use a ‘‘star’’ rating system to judge methodological quality based on three aspects of a study: selection, comparability, and exposure. Scores range from 0 stars (worst) to 9 stars (best), with a score of 5 or higher indicating a moderate-high methodological quality. Two authors independently assessed the quality of included studies. Discrepancies over quality scores were resolved by discussion with all authors and subsequent consensus.
Statistical Analysis
Genotype distributions in the controls were tested for conformation to Hardy-Weinberg equilibrium (HWE) using the chi-square test. HWE in the controls was tested by comparing the expected and observed genotype frequencies using the Pearson chi-square test for goodness of fit. The association between the IL-6 gene polymorphisms and susceptibility to IS was assessed by the pooled odds ratios (ORs) with their corresponding 95% confidence intervals (95%CIs) under three genetic models, including dominant, recessive and allelic model. Taking into consideration possible between-study heterogeneity, a statistical test for heterogeneity was first conducted using Cochran’s Q statistic and the I2 metric. We considered the presence of significant heterogeneity at the 10% level of significance and values of I2 exceeding 50% as an indicator of significant heterogeneity. When no heterogeneity was found with P.0.10 or I2 < 50%, a fixed-effects model was used to estimate the pooled ORs and 95%CIs. Otherwise, a random-effects model was applied. In addition to an overall comparison, stratified analyses, based on ethnicity, source of control, HWE status, and genotyping method where applicable, were also performed to explore possible explanations of between-study heterogeneity and to investigate whether overall reported associations were present in subgroups. Begg’s funnel plot was used to assess the potential for publication bias.
Results
Figure-1 represents a flow diagram of retrieved and excluded studies with their reasons for exclusion. A total of 285 relevant papers were identified using the pre-specified search strategy. In accordance with the inclusion criteria, 16 case-control studies were included for IL-6 G174C with a total of 3317 IS patients and 3482 healthy controls and 3 case control studies for IL-6 G572C with a total of 1814 IS patients and 3219 healthy controls in this meta-analysis. For IL-6 G174C, studies were conducted in two major ethnic populations, with nine including Asians and seven as Caucasians. The publication duration of included studies ranged from 2002 to 2014. The characteristics and methodological quality of all included studies are summarized in Table-1.
Fig. 1:
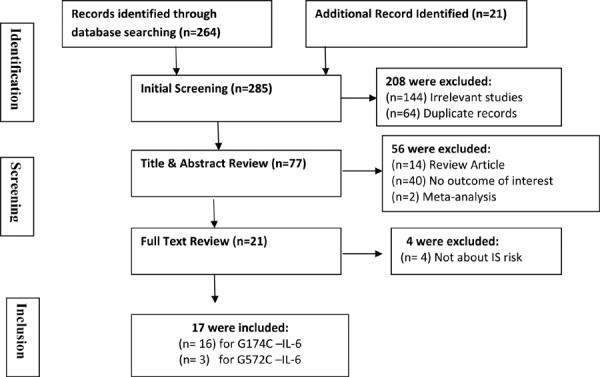
Flow diagram of the selection of studies and specific reasons for exclusion from the present meta-analysis.
Table 1: Characteristic of studies included in the meta-analysis of the association of IL-6 G174C and G572C gene polymorphism with the risk of ischemic stroke.
| S. No | Year | Author | Origin | Ethnicity | Cases/controls | HWE | Matching criteria | Genotyping Method | Variants | M/F | Age[Mean ± S.D] | NOS Star | Source of control |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M = Male; F = Female; S.D = Standard Deviation; HWE = Hardy Weinberg Equilibrium; NOS = Newcastle Ottawa Scale; PB = Population Based; HB = Hospital based, PCR-RFLP-Polymerase Chain Reaction-Restriction Fragment Length Polymorphism, RT-PCR-Real Time-Polymerase Chain Reaction. | |||||||||||||
| 1. | 2010 | Tong et al. | China | Asian | 748/748 | Yes | Age + Sex | Taqman Sequencing | G174C G572C | 431/317 | 61.52 ± 9.68 | 8/9 | PB |
| 431/317 | 60.61 ± 9.11 | ||||||||||||
| 2. | 2005 | Lalouschek et al. | Austria | Caucasian | 404/415 | Yes | Smoking | Multiplex PCR | G174C | 257/147 | 53 (49–57) | 8/9 | PB |
| 253/162 | 49 (43–56) | ||||||||||||
| 3. | 2004 | Flex et al. | Italy | Caucasian | 237/223 | Yes | Age + Sex | PCR-RFLP | G174C | 132/105 | 76.2 ± 9.4 | 6/9 | HB |
| 107/116 | 76.1 ± 6.8 | ||||||||||||
| 4 | 2012 | Tuttolomondo et al. | Italy | Caucasian | 96/48 | No | Age | PCR-RFLP | G174C | 45/51 | 71.9 ± 9.75 | 7/9 | HB |
| 16/32 | 71.4 ± 7.45 | ||||||||||||
| 5. | 2008 | Banerjee et al. | India | Asian | 112/212 | Yes | Age + Sex | PCR-RFLP | G174C | 72/40 | 58.6 ± 14.2 | 7/9 | HB |
| 143/69 | 57.4 ± 8.8 | ||||||||||||
| 6. | 2012 | Chakraborty et al. | India | Asian | 100/120 | Yes | Age + Sex | PCR-RFLP | G174C | 69/31 | 54.0 ± 10.9 | 8/9 | PB |
| 83/37 | 52.5 ± 9.8 | ||||||||||||
| 7. | 2002 | Revilla et al. | Spain | Caucasian | 82/82 | Yes | Age + Sex | PCR-RFLP | G174C | 60/22 | 64.9 ± 9.5 | 7/9 | HB |
| 55/27 | 64.8 ± 9.1 | ||||||||||||
| 8. | 2003 | Ma et al. | China | Asian | 42/18 | Yes | – | – | G174C | 8/9 | PB | ||
| 9. | 2004 | Balding et al. | Ireland | Caucasian | 105/389 | Yes | Sex | PCR-RFLP | G174C | 63/32 | 69 (35–99) | 7/9 | PB |
| 226/163 | 37.1 (18–65) | ||||||||||||
| 10. | 2005 | Chamorro et al. | Spain | Caucasian | 273/105 | Yes | Sex + Same area | PCR-RFLP | G174C | 191/82 | 67.0 ± 10 | 6/9 | HB |
| 62/43 | 64.0 ± 10 | ||||||||||||
| 11. | 2006 | Li et al. | China | Asian | 112/105 | No | NA | PCR-RFLP | G174C | NA | NA | 8/9 | PB |
| 12. | 2010 | Liu et al. | China | Asian | 157/163 | Yes | NA | PCR-RFLP | G174C | NA | NA | 8/9 | PB |
| 13. | 2007 | Huang et al. | China | Asian | 123/88 | Yes | NA | PCR-RFLP | G174C | NA | NA | 8/9 | PB |
| 14. | 2007 | You et al. | China | Asian | 177/112 | Yes | NA | PCR-RFLP | G174C | NA | NA | 7/9 | PB |
| 15. | 2014 | Xuan et al. | China | Asian | 430/461 | Yes | Age | Taqman Sequencing | G174C G572C | 261/169 | 45.4 ± 9.5 | 6/9 | HB |
| 253/208 | 44.8 ± 10.1 | ||||||||||||
| 16. | 2002 | Pola et al. | Italy | Caucasian | 119/133 | Yes | Age + Sex | PCR-RFLP | G174C | 57/62 | 76.8 ± 8.4 | 7/9 | HB |
| 62/71 | 76.2 ± 7.1 | ||||||||||||
| 17. | 2006 | Yamada et al. | Japan | Asian | 636/2010 | Yes | Smoking + BMI | PCR-SSCP | G572C | 372/264 | 67.2 ± 11.1 | 7/9 | HB |
| 844/1166 | 63.0 ± 11.4 | ||||||||||||
Association between the IL-6 G174C Polymorphism and Susceptibility to IS
Sixteen case-control studies28–38 investigated the relationship between G174C and susceptibility to IS with a total of 3317 IS patients and 3432 healthy controls. Since between-study heterogeneity obviously existed (P<0.10 and I2 >50% under all genetic models), the random-effect model was used. As shown in Figure-2, no significant association was found under dominant [GC + CC vs. GG: OR = 1.01, 95% CI: 0.77–1.34, P = 0. 92], recessive [CC vs. GG + GC: OR = 1.02, 95% CI: 0.58–1.78, P = 0. 95] and allelic model [C vs. G Allele: OR = 0.99, 95% CI: 0.74–1.31, P = 0. 93]. Based on an ethnicity stratification analysis, a significant association was observed under a dominant model [GC + CC vs. GG: OR = 1.34, 95% CI: 1.10–1.62, P = 0. 003] in Asians, but not in Caucasians [GC + CC vs. GG: OR = 0.81, 95% CI: 0.55–1.21, P = 0. 31] studies (Figure-3).
Fig. 2:
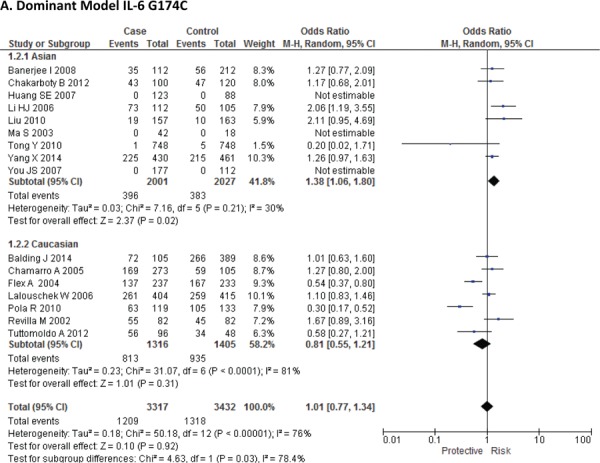
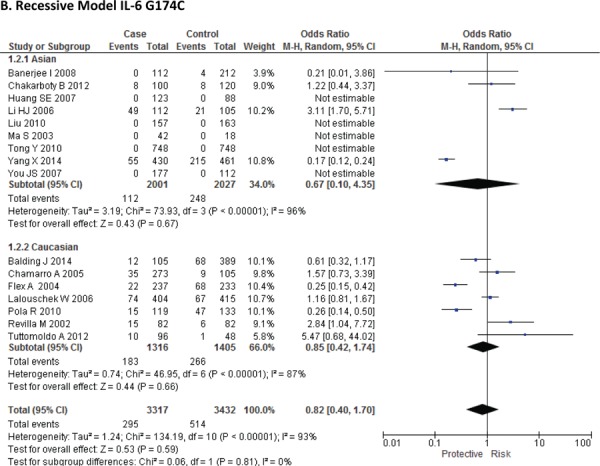
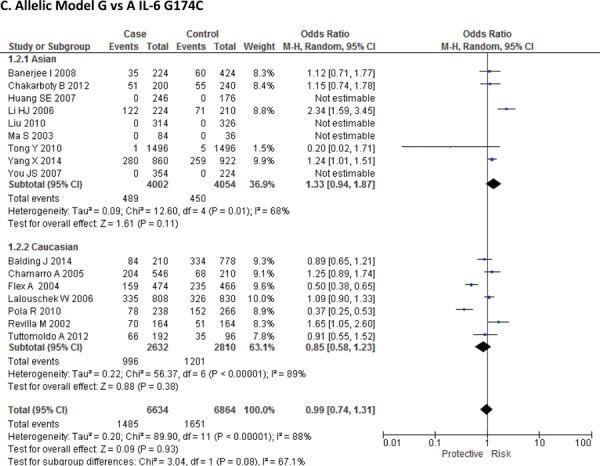
Forest plots for association between IL-6 G174C polymorphism and IS risk in (A) Dominant model (CC + GC vs. GG); (B) Recessive model (CC vs. GG + GC); (C) Allelic Model [C allele vs. G allele] based on ethnic studies.
Fig. 3:
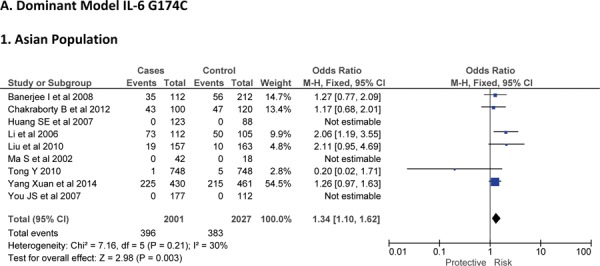
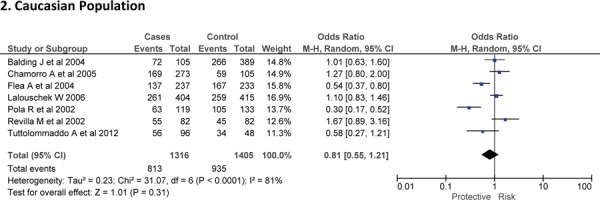
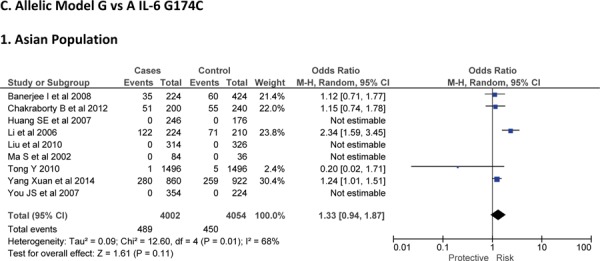
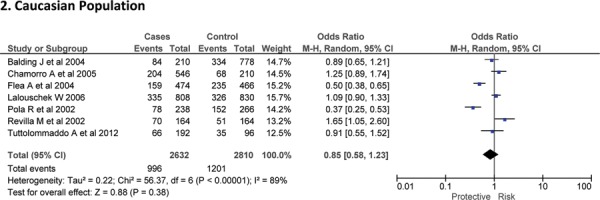
Forest plots for association between IL-6 174 G/C polymorphism and IS risk in (A) Dominant model (CC + GC vs. GG); (B) Recessive model (CC vs. GG + GC); (C) Allelic Model [C allele vs. G allele] based on ethnic studies.
Begg’s funnel plots were used to assess the potential publication bias of included Asian studies under a dominant model for IL-6 G174C SNPs. The shapes of the funnel plots did not reveal any evidence of obvious asymmetry (Figure-4).
Fig. 4:
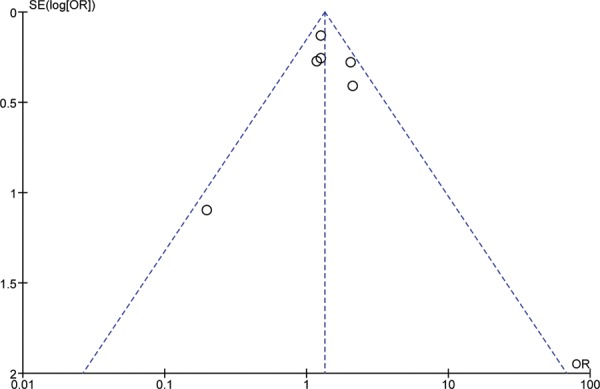
Begg’s Punnel Plot for Publication bias in Asian Population for IL-6 G174C.
Association between the IL-6 G572C Polymorphism and Susceptibility to IS
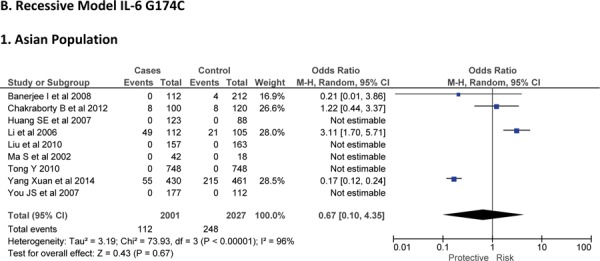
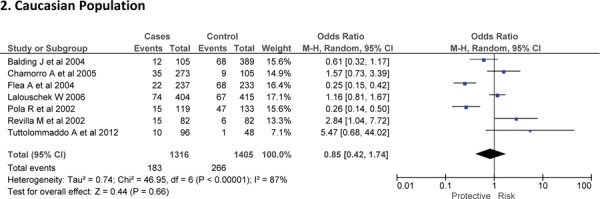
Three case-control studies had investigated the relationship between G572C28,30,41 and susceptibility to IS with a total of 1814 IS patients and 3219 healthy controls. Since between-study heterogeneity obviously existed (P<0.10 and I2 >50% under all genetic models), the random-effects model was used. Overall, the IL-6 G572C polymorphism was not significantly associated with the susceptibility of IS under dominant [GC + CC vs. GG: OR = 0.99, 95% CI: 0.57–1.71, P = 0. 97], recessive [CC vs. GG + GC: OR = 0.93, 95% CI: 0.60–1.45, P = 0. 75] and allelic model [C vs. G Allele: OR = 0.95, 95% CI: 0.66–1.36, P = 0. 76] (Figure-5).
Fig. 5:
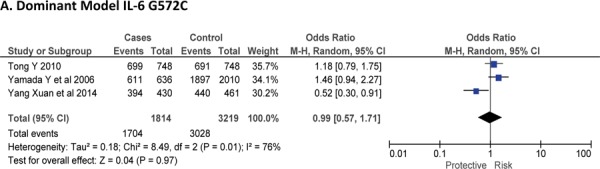
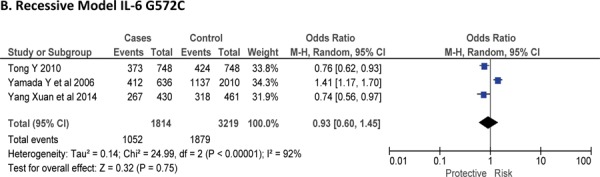
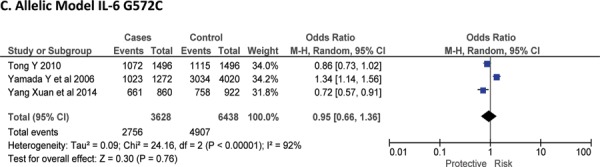
Forest plots for association between IL-6 G572C polymorphism and IS risk in (A) Dominant model (CC + GC vs. GG); (B) Recessive model (CC vs. GG + GC); (C) Allelic Model [C allele vs. G allele].
Discussion
This study was designed to advance the understanding of association between the IL-6 G174C and G572C gene polymorphism and risk of ischaemic stroke. In this meta-analysis, 17 studies (16 studies for G174C, 3 studies for G572C) on IL-6 polymorphisms were performed to provide the most comprehensive assessment of the association between the polymorphisms and IS risk. Our data did not support a genetic association between the polymorphisms and IS risk in populations.
This meta-analysis must be interpreted with caution because of certain limitations. First, the outcomes were based on individual unadjusted ORs, whereas a more precise evaluation should be adjusted by potentially suspected factors, including age, gender, smoking status, and environmental factors. Second, these estimations were obtained by pooling the studies with regard to heterogeneity. However, heterogeneity provided the opportunity to identify factors that modified the genotype. Our meta analysis show heterogeneity except in dominant model of quality studies and some of the factors identified are age, sex, quality of studies and risk factors for ischaemic stroke which could not be stratified in our metaanalysis due to lack of raw data in studies. Pooling studies with different results lead to a high degree of heterogeneity, but might result in hazardous or invalid estimates. Finally, we cannot exclude the possibility that the results were biased because of undetected stratification in the original case–control samples.
In summary, the present meta-analyses did not infer that Ischaemic stroke is a complex multifactorial disease, in addition to genetic factors, environmental factors also play an important role in IS aetiology. Thus, this discrepancy may also be caused by varying geographical distribution, linked to climate, diet, life- style and economic status.
In summary, the present meta-analyses did not support a prominent association of the IL-6 promoter polymorphisms (G174C and G572C) with IS risk. However, the G174C polymorphism might be associated with IS in Asian studies based on sampled studies. More convincing evidence is required to conclude about the relation between these polymorphisms and risk of IS.
Conclusion
Well designed studies are needed to investigate the association of polymorphisms in IL6 larger and various ancestry populations.
Authorship contributions
Pradeep Kumar: Concept, data search, extraction and writing of manuscript, Arun K Yadav: Data search and extraction, Amit Kumar: Analysis, Ram Sagar: Data entry, Awadh K Pandit: Manuscript writing, Kameshwar Prasad: Concept and designing of manuscript.
Footnotes
This article complies with International Committee of Medical Journal editor’s uniform requirements for manuscript.
Conflict of Interests: None: Source of funding: None.
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383(9913):245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol. 2007;6(2):182–187. doi: 10.1016/S1474-4422(07)70031-5. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL. Stroke epidemiology in the developing world. Lancet. 2005;365(9478):2160–2161. doi: 10.1016/S0140-6736(05)66755-4. [DOI] [PubMed] [Google Scholar]
- 4.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J. Leukoc. Biol. 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr FJ, McBride MW, Carswell HVO et al. Genetic aspects of stroke: human and experimental studies. J. Cereb. Blood Flow Metab. 2002;22(7):767–773. doi: 10.1097/00004647-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Matarin M, Brown WM, Dena H . Candidate gene polymorphisms for ischemic stroke. Cereb. In: Stroke J, editor. Circ. 11. Vol. 40. 2009. pp. 3436–3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sehgal PB, Zilberstein A, Ruggieri RM et al. Human chromosome 7 carries the beta 2 interferon gene. Proc. Natl. Acad. Sci. U. S. A. 1986;83(14):5219–5222. doi: 10.1073/pnas.83.14.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zilberstein A, Ruggieri R, Korn JH, Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki S, Tanaka K, Suzuki N. Ambivalent aspects of interleukin-6 in cerebral ischemia: inflammatory versus neurotrophic aspects. J. Cereb. Blood Flow Metab. 29;3:464–479. doi: 10.1038/jcbfm.2008.141. [DOI] [PubMed] [Google Scholar]
- 10.Fishman D, Faulds G, Jeffery R et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J. Clin. Invest. 1998;102(7):1369–1376. doi: 10.1172/JCI2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beamer NB, Coull BM. Clark WM et al. Interleukin-6 and interleukin-1 receptor antagonist in acute stroke. Ann. Neurol. 1995;37(6):800–805. doi: 10.1002/ana.410370614. [DOI] [PubMed] [Google Scholar]
- 12.Fassbender K, Rossol S, Kammer T et al. Proinflammatory cytokines in serum of patients with acute cerebral ischemia: kinetics of secretion and relation to the extent of brain damage and outcome of disease. J. Neurol. Sci. 1994;122(2):135–139. doi: 10.1016/0022-510x(94)90289-5. [DOI] [PubMed] [Google Scholar]
- 13.Kim JS, Yoon SS, Kim YH et al. Serial measurement of interleukin-6, transforming growth factor-beta, and S-100 protein in patients with acute stroke. Stroke J. Cereb. Circ. 1996;27(9):1553–1557. doi: 10.1161/01.str.27.9.1553. [DOI] [PubMed] [Google Scholar]
- 14.Tarkowski E, Rosengren L, Blomstrand C et al. Intrathecal release of pro- and anti-inflammatory cytokines during stroke. Clin. Exp. Immunol. 1997;110(3):492–499. doi: 10.1046/j.1365-2249.1997.4621483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acalovschi D, Wiest T, Hartmann M et al. Multiple levels of regulation of the interleukin-6 system in stroke. Stroke J. Cereb. Circ. 2003;34(8):1864–1869. doi: 10.1161/01.STR.0000079815.38626.44. [DOI] [PubMed] [Google Scholar]
- 16.Castellanos M, Castillo J, García MM et al. Inflammation-mediated damage in progressing lacunar infarctions: a potential therapeutic target. Stroke J. Cereb. Circ. 2002;33(4):982–987. doi: 10.1161/hs0402.105339. [DOI] [PubMed] [Google Scholar]
- 17.Vila N, Castillo J. Dávalos A, et al. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke J. Cereb. Circ. 2000;31(10):2325–2329. doi: 10.1161/01.str.31.10.2325. [DOI] [PubMed] [Google Scholar]
- 18.Greisenegger S, Endler G, Haering D et al. The (-174) G/C polymorphism in the interleukin-6 gene is associated with the severity of acute cerebrovascular events. Thromb. Res. 2003;110(4):181–186. doi: 10.1016/s0049-3848(03)00376-1. [DOI] [PubMed] [Google Scholar]
- 19.Rauramaa R, Väisänen SB, Luong LA et al. Stromelysin-1 and interleukin-6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):2657–2662. doi: 10.1161/01.atv.20.12.2657. [DOI] [PubMed] [Google Scholar]
- 20.Rundek T, Elkind MS, Pittman J et al. Carotid Intima-Media Thickness Is Associated With Allelic Variants of Stromelysin-1, Interleukin-6, and Hepatic Lipase Genes The Northern Manhattan Prospective Cohort Study. Stroke. 2002;33(5):1420–1423. doi: 10.1161/01.STR.0000015558.63492.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McColgan P, Thant KZ, Sharma P. The genetics of sporadic ruptured and unruptured intracranial aneurysms: a genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals. J. Neurosurg. 2010;112(4):714–721. doi: 10.3171/2009.8.JNS092. [DOI] [PubMed] [Google Scholar]
- 22.Caranci F, Briganti F, Cirillo L et al. Epidemiology and genetics of intracranial aneurysms. Eur. J. Radiol. 2013;82(10):1598–1605. doi: 10.1016/j.ejrad.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Basso F, Lowe GDO, Rumley A. et al. Interleukin-6 -174G>C polymorphism and risk of coronary heart disease in West of Scotland coronary prevention study (WOSCOPS). Arterioscler. Thromb. Vasc. Biol. 2002;22(4):599–604. doi: 10.1161/01.atv.0000013283.84306.1a. [DOI] [PubMed] [Google Scholar]
- 24.Flex A, Gaetani E, Pola R et al. The -174 G/C polymorphism of the interleukin-6 gene promoter is associated with peripheral artery occlusive disease. Eur. J. Vasc. Endovasc. Surg. Off. J. Eur. Soc. Vasc. Surg. 2002;24(3):264–268. doi: 10.1053/ejvs.2002.1711. [DOI] [PubMed] [Google Scholar]
- 25.Pola R, Gaetani E, Flex A et al. -174 G/C interleukin-6 gene polymorphism and increased risk of multi-infarct dementia: a case-control study. Exp. Gerontol. 2002;37(7):949–955. doi: 10.1016/s0531-5565(02)00031-1. [DOI] [PubMed] [Google Scholar]
- 26.Chapman CML, Beilby JP, Humphries SE et al. Association of an allelic variant of interleukin-6 with subclinical carotid atherosclerosis in an Australian community population. Eur. Heart J. 2003;24(16):1494–1499. doi: 10.1016/s0195-668x(03)00313-0. [DOI] [PubMed] [Google Scholar]
- 27.Jones KG, Brull DJ, Brown LC et al. Interleukin-6 (IL-6) and the Prognosis of Abdominal Aortic Aneurysms. Circulation. 2001;103(18):2260–2265. doi: 10.1161/01.cir.103.18.2260. [DOI] [PubMed] [Google Scholar]
- 28.Yamada Y, Metoki N, Yoshida H et al. Genetic risk for ischemic and hemorrhagic stroke. Arterioscler. Thromb. Vasc. Biol. 2006;26(8):1920–1925. doi: 10.1161/01.ATV.0000229694.97827.38. [DOI] [PubMed] [Google Scholar]
- 29.Tuttolomondo A, Di Raimondo D, Forte GI et al. Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine. 2012;58(3):398–405. doi: 10.1016/j.cyto.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Feng L, Li C. et al. Association of IL-6-174G>C and -572C>G polymorphisms with risk of young ischemic stroke patients. Gene. 2014;539(2):258–262. doi: 10.1016/j.gene.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 31.Balding J, Livingstone WJ, Pittock SJ et al. The IL-6 G-174C polymorphism may be associated with ischaemic stroke in patients without a history of hypertension. Ir. J. Med. Sci. 2004;173(4):200–203. doi: 10.1007/BF02914551. [DOI] [PubMed] [Google Scholar]
- 32.Banerjee I, Gupta V, Ahmed T et al. Inflammatory system gene polymorphism and the risk of stroke: a case-control study in an Indian population. Brain Res. Bull. 2008;75(1):158–165. doi: 10.1016/j.brainresbull.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Chakraborty B, Chowdhury D, Vishnoi G et al. Interleukin-6 Gene -174 G/C Promoter Polymorphism Predicts Severity and Outcome in Acute Ischemic Stroke Patients from North India. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2012 doi: 10.1016/j.jstrokecerebrovasdis.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Chamorro A, Revilla M, Obach V et al. The -174G/C polymorphism of the interleukin 6 gene is a hallmark of lacunar stroke and not other ischemic stroke phenotypes. doi:10.1159/000082785. Cerebrovasc. Dis. Basel Switz. 2005;19(2):91–95. doi: 10.1159/000082785. [DOI] [PubMed] [Google Scholar]
- 35.Liu D.F., Chen N.Y., Wang D.L., Zhang C.X., Zhang B.Q. Research on the polymorphism of IL-6 −174G/C gene of patients with cerebral infarction among Han people in Tangshan areas. Mod. Prev. Med. 2010;37:3419–3427. [Google Scholar]
- 36.Li H.J. Synergistic Effect of−174G/C Polymorphism of the Interleukin-6 Gene Promoter and 469 E/K Polymorphism of the Intercellular Adhesion Molecule-1 Gene in Patients with Cerebral Infarction. Qidao University 1–42 (Master thesis) 2006 [Google Scholar]
- 37.Huang S.E. The Association of Inflammatory Cytokines Gene Polymorphisms With Atherothrombotic Cerebral Infarction. Fujian Medical University 1–39 (Master thesis) 2005 [Google Scholar]
- 38.You J.S., Huang P.X., Liu M.C. et al. Study of ICAM-1Gene K469E Polymorphism and IL-6 Gene −174G/C Polymorphism in Cerebral Infarction Patients. Chin. J. Gerontol. 2007;27:1580–1582. [Google Scholar]
- 39.Ma S., Jiang L.Y., Wu et al. Relationship between G to C polymorphism at −174 of the interleukin-6 gene and acute cerebral infarction. J. ApopLexy Nerv. Dis. 2003;20:152–154. [Google Scholar]
- 40.Lalouschek W, Schillinger M, Hsieh K et al. Polymorphisms of the inflammatory system and risk of ischemic cerebrovascular events. Clin. Chem. Lab. Med. CCLM FESCC. 2006;44(8):918–923. doi: 10.1515/CCLM.2006.165. [DOI] [PubMed] [Google Scholar]
- 41.Tong Y, Wang Z, Geng Y et al. The association of functional polymorphisms of IL-6 gene promoter with ischemic stroke: analysis in two Chinese populations. Biochem. Biophys. Res. Commun. 2010;391(1):481–485. doi: 10.1016/j.bbrc.2009.11.084. [DOI] [PubMed] [Google Scholar]
- 42.Lalouschek W, Schillinger M, Hsieh K et al. Polymorphisms of the inflammatory system and risk of ischemic cerebrovascular events. Clin. Chem. Lab. Med. CCLM FESCC. 2006;44(8):918–923. doi: 10.1515/CCLM.2006.165. [DOI] [PubMed] [Google Scholar]
- 43.Revilla M, Obach V, Cervera A et al. A -174G/C polymorphism of the interleukin-6 gene in patients with lacunar infarction. Neurosci. Lett. 2002;324(1):29–32. doi: 10.1016/s0304-3940(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 44.Moher D, Liberati A, Tetzlaff J et al. PRISMA GroupPreferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]


