Figure 2. Solution structure of the anti-HIV protein OAA samples the bound conformation.
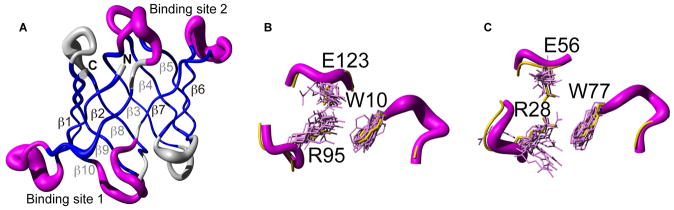
A, The overall fold of the sugar-free solution structure of OAA comprises 10 anti-parallel β-strands (blue) that form a β-barrel, very similar to the sugar-free and sugar-bound X-ray structures (PDB code 3S5V and 3S5X, [3] respectively). The two sugar binding sites (magenta; details of binding site 1 and 2 conformations are shown in B and C, respectively), resemble the sugar-bound conformation of the X-ray structure (yellow). Side chains, directly involved in the carbohydrate binding (W10, R95 and E123 in site 1 and R28, E56 and W77 in site 2) are shown in stick representation. The mean position of the backbone Cα atoms is shown in tube representation, with the radius of the tube corresponding to the average deviation of all conformers with respect to the mean.
