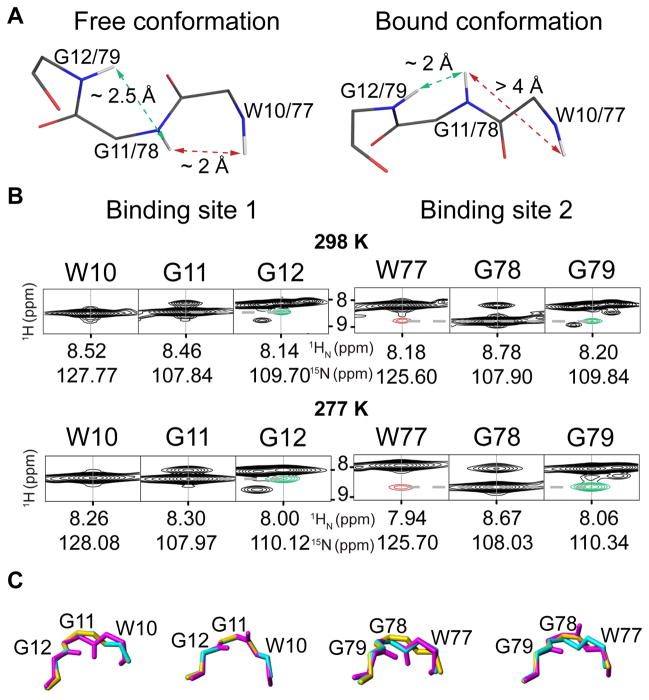
Figure 3. Backbone amide distances measured in solution by NMR are sensitive measures of a flipped peptide bond.
A, Stick representations of the backbone conformations in the sugar-free and sugar-bound crystal structure (PDB code 3S5V and 3S5X, [3] respectively). A short distance (2.2 Å) between the backbone amide protons (HN) of W77 and G78 in site 2 (dashed red arrows) in the sugar-free conformation is longer (4.4 Å) in the sugar bound conformation. Binding site 1 displays the sugar-bound conformation in the sugar-free crystal structure due to protein-protein contacts within the crystal. B, NOE cross peaks corresponding to the distances depicted in A for different temperatures (298 K, top; 277 K, bottom) between HN W77 and HN G78 (red) and between HN G11/G78 and HN G12/G79 (green). The intensity ratio between these NOE cross peaks indicates that both the sugar-free and the sugar-bound conformations are present. C, The different backbone conformations sampled by OAA in solution (magenta) match the sugar-free (cyan) and sugar-bound (yellow) conformations seen in the X-ray structures in both binding sites.