Fig. 2.
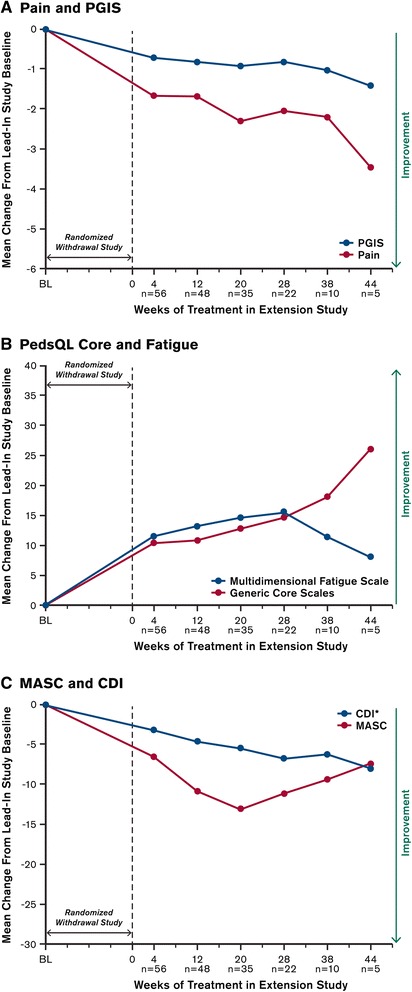
Mean changes from baseline in outcome measures during the extension study. Baseline was defined as pre-treatment values (i.e., patients’ scores prior to receiving the first dose of milnacipran in the open-label period of the randomized withdrawal study). For each visit in the extension study, baseline only includes pre-treatment values for those patients who completed that particular study visit. The n-values represent numbers of patients with valid assessments at baseline and at each extension study visit; graph only includes study visits that had >1 patient. *CDI was primarily used as a safety outcome. CDI = Children’s Depression Inventory, MASC = Multidimensional Anxiety Scale for Children, PedsQL = Pediatric Quality of Life Inventory, PGIS = Patient Global Impression of Severity
