Abstract
Background
Conditioned fear memories can be updated by extinction during reconsolidation, and this effect is specific to the reactivated conditioned stimulus (CS). However, a traumatic event can be associated with several cues, and each cue can potentially trigger recollection of the event. In the present study, we introduced a technique to target all diverse cues associated with an aversive event that causes fear.
Methods
In the human experiments, the subjects underwent modified fear conditioning, in which they were exposed to an unconditioned stimulus (US) or unreinforced CS to reactivate the memory and then underwent extinction, spontaneous recovery, and reinstatement. In the animal experiments, rats underwent contextual fear conditioning under a similar protocol as the used in the human experiments. We also explored the molecular alterations after US reactivation in rats.
Results
We found that presentation of a lower-intensity US following extinction disrupted the associations between the different CSs and reactivated US in both humans and rats. This disruptive effect persisted for at least 6 months in humans and was selective to the reactivated US. This procedure was also effective for remote memories in both humans and rats. Compared with the CS, the US induced stronger endocytosis of AMPA glutamate receptors 1 and 2 and stronger activation of protein kinase A, p70S6 kinase, and cyclic adenosine monophosphate response element binding protein in the dorsal hippocampus in rats.
Conclusions
These findings demonstrate that a modified US retrieval-extinction strategy may have a potential impact on therapeutic approaches to prevent the return of fear.
Keywords: fear memory, unconditioned stimulus, retrieval, extinction, reconsolidation, hippocampus
Introduction
Currently, anxiety disorders are often treated by exposure therapy that extinguishes or suppresses fear responses by repeatedly exposing the subjects to the fear-inducing stimulus without harmful consequences (1, 2). This has been successfully modeled in both humans and animals using Pavlovian fear conditioning and extinction, in which an originally neutral conditioned stimulus (CS) is associated with a noxious unconditioned stimulus (US), and the fear response is extinguished after repeated exposure to the CS without the US (3, 4). However, although exposure therapy in individuals with anxiety disorders initially reduces fear responses, the reduced fear often returns in some conditions (5, 6). The reemergence of extinguished fear indicates that extinction normally leaves the original memory intact, which limits the long-term effectiveness of exposure therapy (5).
Exposure to a reminder of the conditioning experience, so that a memory is putatively re-encoded during a process termed “reconsolidation,” has been shown to make a memory temporarily susceptible to disruption by several manipulations (7–9). Pharmacological treatments have been used to disrupt reconsolidation for more than a decade (10–12), but side-effects and the typical intracranial route of administration make these drugs more suitable for animal research than human treatment. Research has recently showed that a drug-free procedure that uses reconsolidation to make extinction more effective disrupted both aversive and appetitive memories in both animals and humans (13–18). However, extinction during reconsolidation permanently affects only memory for the reactivated CS and does not interfere with memory for other cues associated with the original learning event (16). A traumatic event is usually associated with several different cues, and each potentially triggers recollection of the event and elicits a fear reaction. Therefore, eliminating fear responses to all cues associated with the traumatic event through behavioral interference of reconsolidation is desirable. Presenting the US alone before extinction to trigger and disrupt US-specific reconsolidation could represent a promising avenue for treatment because pharmacological manipulations after US-triggered reconsolidation can disrupt the conditioned memory for multiple CSs associated with that US but not with other USs (19). This specificity could make it useful to disrupt unhealthy emotional memories, while leaving other adaptive aversive memories intact. We investigated whether US-retrieval extinction can disrupt representations of the US and persistently eliminate all US-associated memory traces and responses.
Methods and Materials
Human experiments
Each participant signed a consent form approved by the Institutional Review Board of Peking University and was paid for their participation.
Fear conditioning acquisition
We used a modified fear conditioning procedure (16). In all of the experiments, the CS+ was paired with an electric shock (US) on a partial reinforcement schedule (38% reinforced). In Experiment 1, one CS was paired with the US (paired CS+ or unpaired CS− with US). In Experiments 2, 3, and 5, two distinct CSs were paired with the same US (paired CS1+ and CS2+ or unpaired CS− with US). In Experiment 6, each of two distinct CSs was paired with the different US respectively (paired CS1+ with US1, paired CS2+ with US2, and unpaired CS−). See Methods and Materials and Table S1 in Supplement 1 for additional information.
Reactivation and extinction
In reactivation and extinction, all of the CSs were nonreinforced. In the animal experiment, we found that extinction after strong US reactivation, in which the intensity was same as the one used during acquisition, could not extinguish the fear response in rats (Figure. S1 in Supplement 1). Therefore, during US reactivation in humans, a weaker electric shock, in which the intensity was half of the one used during acquisition (200 ms), was administered. During CS reactivation, CS+ was presented once. During extinction, 10 CS+ and 10 CS− were presented. See Methods and Materials and Table 1 in Supplement 1 for additional details.
Test
In the test, all of the CSs were nonreinforced. The spontaneous recovery test in Experiments 1–3, 5, and 6 occurred 24 h after the end of extinction. At the end of the spontaneous recovery test (Experiments 1–3, 5, and 6) or the end of extinction (Experiment 4), the response to the CS was thoroughly extinguished, and the participants then received three unsignaled USs. The reinstatement test was following by the unsignaled USs. See Methods and Materials and Table 1 in Supplement 1 for additional information.
Psychophysiological stimulation and assessment
Electric shock was delivered by a constant-current stimulator via a STM 200 stimulator (BIOPAC Systems, Goleta, CA, USA). A stimulating electrode was attached to the right inner wrist or the right eyelids. Stimulus presentation was controlled by a computer using E-Prime software. Conditioning was assessed in terms of the skin conductance response (SCR), which was measured using a Biopac MP150 system and analyzed using Acknowledge software (BIOPAC Systems, Goleta, CA, USA). See Methods and Materials in Supplement 1 for additional details.
Statistical analysis
We conducted mixed-factor analyses of variance (ANOVAs) for Experiments 1–4 and a repeated-measures ANOVA for Experiments 5 and 6. To assess expectation of the reinforcer, only nonreinforced trials of the CS+ were included in the analysis. The differential fear response was assessed by subtracting the responses to the CS− from the responses to the CS+ in corresponding trials. Subjects that showed successful levels of fear acquisition and extinction were included in the analysis (see Methods and Materials in Supplement 1). Two-tailed tests and an alpha level of .05 were used for all of the statistical comparisons.
Rat experiments
The fear conditioning procedure in rats was based on the work of Lubin and Sweatt (20), with some modifications. For added details on the procedures, see the Materials and Methods in Supplement 1.
Results
Extinction interfered with the reconsolidation triggered by US presentation
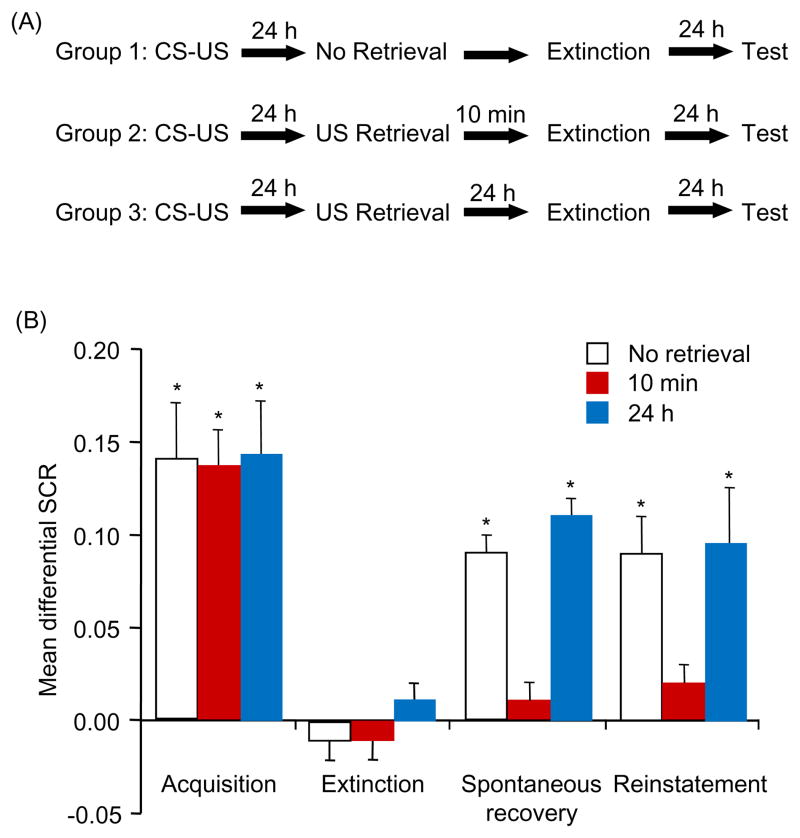
We first examined whether extinction during US retrieval-triggered reconsolidation can disrupt fear memory in Experiment 1 (Figure. 1A). All of the participants in the three groups (10 min, 24 h, and no retrieval) achieved successful acquisition [F(1,51) = 98.04, p < .05] and extinction [F(1,51) = 86.66, p < .05]. No significant difference was found between groups in either acquisition or extinction (all p > .05).
Figure 1.
Extinction 10 min after US exposure prevented spontaneous recovery and reinstatement of extinguished fear in humans. (A) Experimental design and timeline. (B) Mean differential SCR (CS+ minus CS−) during acquisition (late phase), extinction (last trial), test for spontaneous recovery (first trial), and reinstatement (first trial) for each of the experimental groups (10 min, 24 h and no retrieval). Spontaneous recovery (first trial of this test vs. last trial of extinction) and reinstatement (first trial of reinstatement vs. last trial of spontaneous recovery) were found in the 24 h and no-retrieval groups. No spontaneous recovery or reinstatement was found in the 10 min group. *p < .05, comparisons between acquisition and extinction, between extinction and the first trial of spontaneous recovery, and between last trial of spontaneous recovery and reinstatement (all within-group). The data are expressed as mean ± SEM (n = 16–19 per group). CS, conditioned stimulus; US, unconditioned stimulus; SCR, skin conductance response.
Spontaneous recovery was assessed using a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factor Test (first trial of spontaneous recovery vs. last trial of extinction). This analysis showed main effects of Group [F(2,51) = 29.56, p < .05] and Test [F(1,51) = 102.60, p < .05] and a significant interaction [F(2,51) = 14.05, p < .05]. The post hoc analysis showed that spontaneous recovery occurred in the no-retrieval group and 24 h group (both p < .05) but not in the 10 min group (p > .05). A one-way ANOVA showed that fear responses in the last trial of spontaneous recovery in all of the groups were similar (p > .05).
During reinstatement, a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factor Test (last trial of spontaneous recovery vs. first trial of reinstatement), showed main effects of Test [F(1,51) = 80.44, p < .05] and Group [F(2,51) = 17.52, p < .05] and a significant interaction [F(2,51) = 13.79, p < .05]. Follow-up t-tests showed that only the fear response in the 10 min group was not reinstated (p > .05; Figure. 1B). Additionally, we found that extinction 10 min after US exposure prevented the spontaneous recovery and reinstatement of extinguished fear in rats (Figure. S2 in Supplement 1). Altogether, these findings showed that extinction during US-triggered reconsolidation, similar to extinction during CS-triggered reconsolidation (16), prevented the return of fear responses.
US-triggered reconsolidation targets all CSs
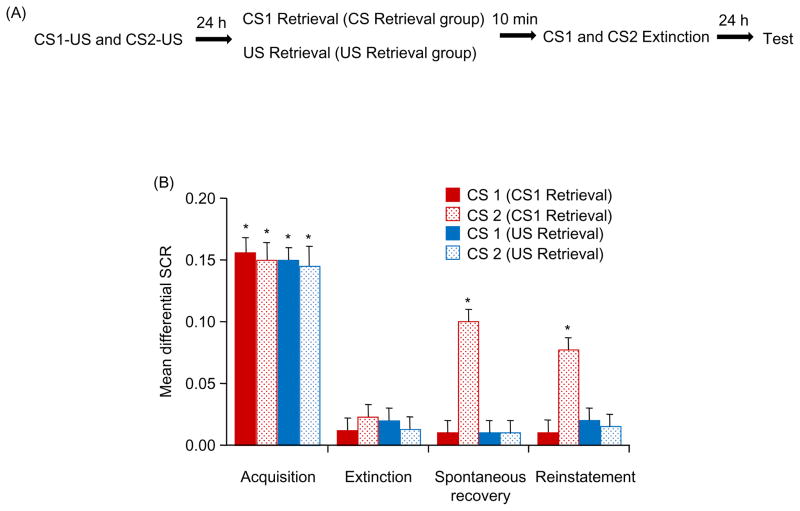
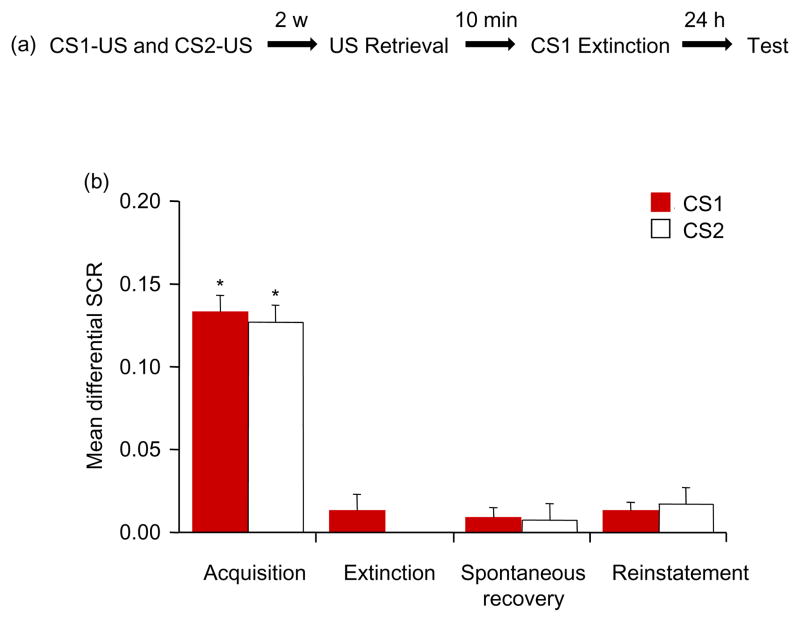
Previous studies indicated that extinction during CS-triggered reconsolidation permanently affects only memory of the reactivated CS (14–16). In Experiment 2, we investigated whether US exposure destabilizes multiple CS-US associations that are conditioned to the reactivated US (Figure. 2A). All of the participants in the two groups (US retrieval and CS retrieval) achieved successful acquisition [F(1,34) = 22.71, p < .05] and extinction [F(1,34) = 24.78, p < .05]. No significant difference was found between groups in either acquisition or extinction (all p > .05).
Figure 2.
Extinction after exposure to the US alone disrupted the reconsolidation of fear conditioning in response to both the CS1 and CS2 in humans. (A) Experimental design and timeline. (B) Mean differential SCR (CS1+ minus CS− or CS2+ minus CS−) during acquisition (late phase), extinction (last trial), spontaneous recovery test (first trial), and reinstatement (first trial). *p < .05, comparisons between acquisition and extinction, between extinction and the first trial of the spontaneous recovery test, and between the last trial of the spontaneous recovery test and reinstatement (all within-group). The data are expressed as mean ± SEM (n = 18 per group).
Spontaneous recovery was assessed using a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factors CS+ (CS1+ and CS2+) and Test (first trial of spontaneous recovery vs. last trial of extinction). This analysis showed main effects of Group [F(1,34) = 7.91, p < .05], CS+ [F(1,34) = 9.49, p < .05), and Test [F(1,34) = 4.63, p < .05] and a significant Group × CS+ × Test interaction [F(1,34) = 5.30, p < .05]. The post hoc analysis showed that significant spontaneous recovery occurred for the CS2+ in the CS retrieval group (p < .05). However, no spontaneous recovery for the CS1+ and CS2+ in the US retrieval group or CS1+ in the CS retrieval group was found (all p > .05). A mixed-factor ANOVA showed that fear responses (CS1+ and CS2+) in the last trial of spontaneous recovery in both groups was similar (all p > .05).
During reinstatement, a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factors CS+ (CS1+ and CS2+) and Test (last trial of spontaneous recovery vs. first trial of reinstatement), showed main effects of Group [F(1,34) = 4.31, p < .05], CS+ [F(1,34) = 17.38, p < .05], and Test [F(1,34) = 8.38, p < .05] and a significant Group × CS+ × Test interaction [F(1,34) = 16.94, p < .05]. The post hoc analysis showed significant reinstatement of conditioned fear for the CS2+ (p < .05) but not CS1+ (p > .05) in the CS retrieval group. No reinstatement was found in the US retrieval group (all p > .05; Figure. 2B). These findings showed that the extinction of multiple CSs during US-triggered reconsolidation inhibited the fear response to each CS.
Disruption of US-triggered reconsolidation of memory for multiple CSs by extinction of a single CS
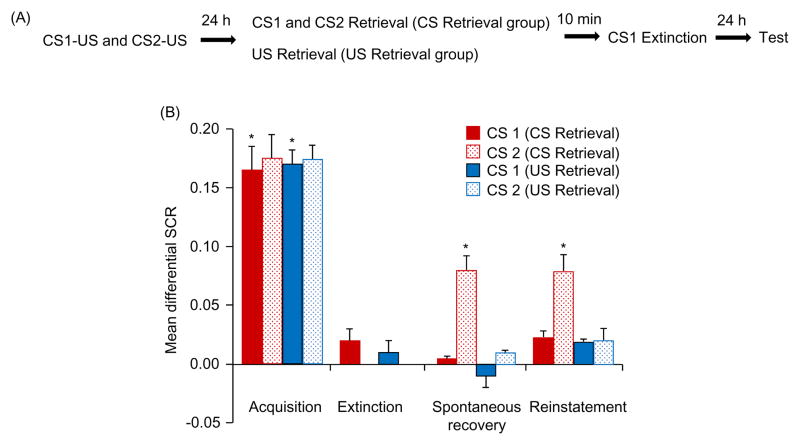
In Experiment 3, we investigated whether interfering with US-triggered reconsolidation using one fear-predictive cue affects the fate of another cue conditioned to the reactivated US (Figure. 3A). All of the participants in the two groups (US retrieval and CS retrieval) achieved successful acquisition [F(1,35) = 50.02, p < .05] and extinction [F(1,35) = 33.12, p < .05]. No significant difference was found between groups in either acquisition or extinction (all p > .05).
Figure 3.
Either CS1 or CS2 extinction following exposure to the US alone disrupted the reconsolidation of fear conditioning in response to both the CS1 and CS2 in humans. (A) Experimental design and timeline. (B) Mean differential SCR (CS1+ minus CS− or CS2+ minus CS−) during acquisition (late phase), extinction (last trial), spontaneous recovery test (first trial), and reinstatement (first trial). *p < .05, comparisons between acquisition and extinction, between the last trial of extinction (CS1+) and first trial of spontaneous recovery (CS1+ and CS2+), and between the last trial of spontaneous recovery and reinstatement (all within-group). The data are expressed as mean ± SEM (n = 18–19 per group).
Spontaneous recovery was assessed using a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factor Test (CS1+ during last trial of extinction vs. CS1+ and CS2+ during first trial of spontaneous recovery). This analysis showed main effects of Group [F(1,35) = 12.76, p < .05] and Test [F(2,35) = 20.03, p < .05] and a significant interaction [F(2,35) = 12.79, p < .05]. The post hoc analysis showed that only fear responses to the CS2+ recovered in the CS retrieval group (p < .05). A mixed-factor ANOVA showed that fear responses (both CS1+ and CS2+) in the last trial of spontaneous recovery in both groups was similar (all p > .05).
During reinstatement, a mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factors CS+ (CS1+ and CS2+) and Test (last trial of spontaneous recovery vs. first trial of reinstatement) was used. This analysis revealed main effects of Group [F(1,35) = 8.33, p < .05], CS+ [F(1,35) = 5.91, p < .05], and Test [F(1,35) = 15.06, p < .05] and Group × Test [F(1,35) = 5.71, p < .05], CS+ × Test [F(1,35) = 7.36, p < .05], and Group × CS+ [F(1,35) = 5.47, p < .05] interactions. The post hoc analysis revealed significant reinstatement of the fear response to the CS2+ in the CS retrieval group (p < .05) but not for the CS1+ in the CS retrieval group or both CSs+ in the US retrieval group (all p > .05; Figure. 3B). In the rat experiment, we also found that either CS1 or CS2 extinction during the US-triggered reconsolidation period permanently disrupted fear conditioning to both the CS1+ and CS2+ (Figure. S3 in Supplement 1). These results indicate that the extinction of a single CS during the US-triggered reconsolidation time window was sufficient to block subsequent fear responses to all US-related CSs.
To determine the possible mechanisms of US- and CS-triggered fear reconsolidation, we evaluated alterations of several plasticity-related proteins (PRPs) in the rat dorsal hippocampus after US or CS retrieval. Some PRPs changes in the dorsal hippocampus have been previously shown to be involved in the reconsolidation of contextual fear memory (21–23). Our results showed that the endocytosis of glutamate receptor subunits GluR1 and GluR2 (21) and activation of protein kinase A, p70S6 kinase, and cyclic adenosine monophosphate response element binding protein (22, 23) in the dorsal hippocampus induced by US retrieval in rats were stronger than those induced by CS retrieval. Additionally, the extracellular signal-regulated kinase/brain-derived neurotrophic factor pathway, which is known to play a role in the consolidation but not reconsolidation of contextual fear memory (25, 26), was unaffected by either CS or US retrieval. Furthermore, CS or US presentations alone did not produce PRPs alteration in dorsal hippocampus in naïve rats demonstrating that the molecular alterations seen were not due to the intrinsic aversiveness of the US apart from its role in reactivating the fear memory. The altered molecules induced by US retrieval recovered to the normal level 24 h later. (Figure S4 in Supplement 1). These results suggest that US exposure triggered stronger molecular alterations in hippocampal neurons to cause the fear memory to be updated or erased.
Blockade of conditioned fear is maintained for at least 6 months
In Experiment 4, approximately 6–7 months later, the participants from Experiment 3 were invited to return to the laboratory to assess whether the blockade of memory persisted. Twenty-four of the participants from Experiment 3 participated in the follow-up study. These participants from two groups (US retrieval and CS retrieval) were first exposed to re-extinction and then underwent the reinstatement test. A mixed-factor ANOVA showed that the fear responses (both CS1+ and CS2+) in the last trial of re-extinction in both groups were similar (all p > .05). A mixed-factor ANOVA, with the between-subjects factor Group and within-subjects factors CS+ (CS1+ and CS2+) and Test (last trial of re-extinction vs. first trial of reinstatement), revealed significant main effects of Group [F(1,22) = 12.98, p < .05], CS+ [F(1,22) = 28.44, p < .05], and Test [F(1,22) = 31.63, p < .05] and a significant Group × CS+ × Test interaction [F(1,22) = 26.50, p < .05]. The post hoc analysis revealed significant reinstatement of conditioned fear to the CS2+ (p < .05) but not CS1+ (p > .05) in the CS retrieval group. No reinstatement was found for either CS+ in the US retrieval group (all p > .05; Figure. 4). These results indicate that US-retrieval extinction led to long-lasting blockade of the return of fear.
Figure 4.
Persistence of blockade of fear conditioning in response to both the CS1 and CS2 by either CS1 or CS2 extinction following exposure to the US alone in humans. The reinstatement index is the mean differential SCR (CS1+ minus CS− or CS2+ minus CS−) during reinstatement (first trial) after re-extinction 6 months later. Significant reinstatement of conditioned fear was found for the CS2+ but not CS1+ in the CS retrieval group. No reinstatement was found in the US retrieval group. *p < .05. The data are expressed as mean ± SEM (n = 11–13 per group).
US retrieval-extinction procedure disrupts remote fear memory
In Experiment 5, we investigated whether the US retrieval-extinction procedure has disruptive effects on remote fear memory. Two weeks after fear conditioning, the subjects underwent a US retrieval-extinction procedure. Tests for spontaneous recovery and reinstatement were conducted 24 h later (Figure. 5A). All of the participants achieved successful acquisition [F(1,14) = 58.51, p < .05] and CS1+ extinction (t14 = 9.63, p < .05). No significant difference was found between groups in either acquisition or extinction (all p > .05).
Figure 5.
The US retrieval-extinction procedure disrupted remote fear memory in humans. (A) Experimental design and timeline. (B) Mean differential SCR (CS+ minus CS−) during acquisition (late phase), extinction (last trial), test for spontaneous recovery (first trial), and reinstatement (first trial). No spontaneous recovery or reinstatement was found. *p < .05, comparison between acquisition and extinction (within-group). The data are expressed as mean ± SEM (n = 15).
Spontaneous recovery was assessed using a repeated-measures ANOVA, with the within-subjects factor Test (CS1+ during last trial of extinction vs. CS1+ and CS2+ during first trial of spontaneous recovery). This analysis showed no significant effects [F(1,14) = 0.32, p > .05]. A paired t-test showed that the responses to the CS1+ and CS2+ were similar before reinstatement (p > .05).
For the reinstatement test, a repeated-measures ANOVA, with the within-subjects factors CS+ (CS1+ and CS2+) and Test (last trial of spontaneous recovery vs. first trial of reinstatement), was conducted. This analysis showed no effect of CS+ [F(1,14) = 0.28, p =.61] or Time [F(1,14) =.12, p =.73] and no interaction [F(1,14) =.98, p =.34; Figure. 5B]. In the rat experiment, we also found that applying US-retrieval extinction 2 weeks after fear conditioning prevented the return of fear memory (Figure. S5 in Supplement 1). Altogether, these results indicate that the US retrieval-extinction procedure was effective for long-lasting fear memory.
Reconsolidation is specific to the reactivated US
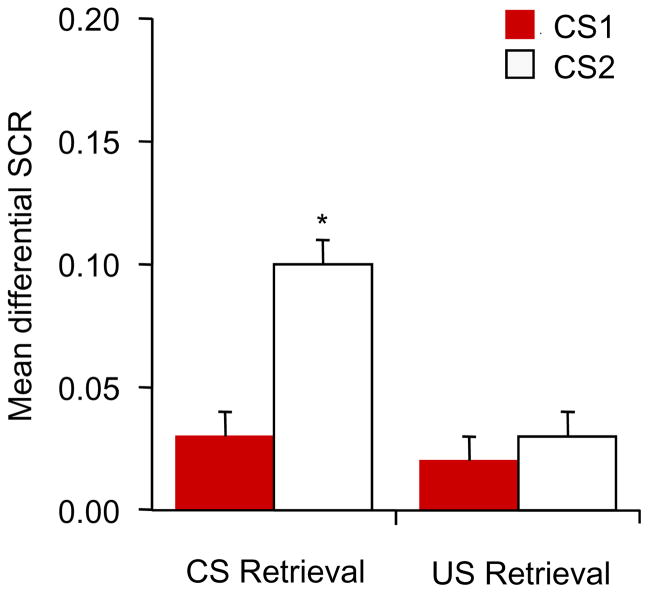
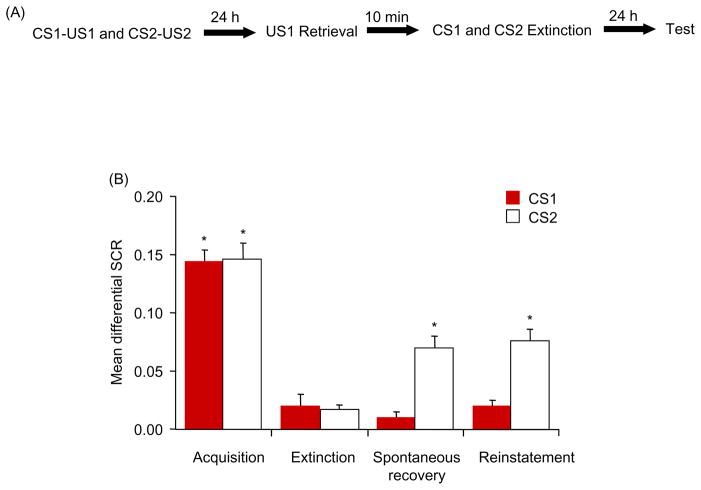
Considering that behaviorally interfering with US-triggered reconsolidation may be clinically useful, determining whether the impairment is specific to the reactivated US is important. In Experiment 6, we investigated whether the disruption of reconsolidation is selective for the reactivated US (Figure. 6A). All of the participants achieved successful acquisition [F(1,18) = 77.35, p < .05] and extinction [F(1,18) = 78.7, p < .05]. The responses to the CS1+ and CS2+ in all of the participants were similar in both acquisition and extinction (all p > .05).
Figure 6.
Reconsolidation is specific to the reactivated US. (A) Experimental design and timeline. (B) Mean differential SCR (CS1+ minus CS− or CS2+ minus CS−) during acquisition (late phase), extinction (last trial), spontaneous recovery test (first trial), and reinstatement (first trial). *p < .05, comparisons between acquisition and extinction, between extinction and the first trial of spontaneous recovery, and between the last trial of the spontaneous recovery test and reinstatement (all within-group). The data are expressed as mean ± SEM (n = 19).
Spontaneous recovery was assessed using repeated-measures ANOVA, with the within-subjects factors CS+ (CS1+ and CS2+) and Test (first trial of spontaneous recovery vs. last trial of extinction). This analysis showed main effects of CS+ [F(1,18) = 26.34, p < .05] and Test [F(1,18) = 8.98, p < .05] and a significant interaction [F(1,18) = 18.4, p < .05). The post hoc analysis indicated that subjects showed spontaneous recovery of fear response to the CS2+ (p < .05) but not CS1+ (p > .05). A paired t-test (CS1+ and CS2+) showed that fear responses to the CS1+ and CS2+ in the last trial of spontaneous recovery were similar (p > .05).
The reinstatement test was analyzed using a repeated-measures ANOVA, with the within-subjects factors CS+ (CS1+ and CS2+) and Test (last trial of spontaneous recovery vs. first trial of reinstatement). This analysis revealed significant main effects of CS+ [F(1,18) = 15.98, p < .05] and Test [F(1,18) = 104.32, p < .05] and a significant interaction [F(1,18) = 21.79, p < .05]. The post hoc analysis revealed significant reinstatement of fear responses to the CS2+ (p < .05) but not CS1+ (p > .05; Figure. 6B). These results indicate that the effect of extinction during the US-triggered reconsolidation time window on subsequent fear responses to the CSs was US-specific, and the procedure only persistently blocked the fear response related to the reactivated US.
Discussion
The US is a powerful reminder that is able to trigger reconsolidation (19). Consistent with previous reports (19, 24), our results demonstrate that US presentation can reactivate a memory trace and make it labile, so that extinction can disrupt the consolidated fear memory. Importantly, this US retrieval-extinction is effective for not only recent but also remote fear memory. Our US retrieval-extinction findings extend previous findings, in which a CS retrieval-extinction procedure impaired fear memory for that CS (15, 16). The US retrieval-extinction strategy has potential impact for therapeutic approaches to prevent the return of fear.
Interestingly, we also found that extinction of one fear-predictive cue during US-triggered reconsolidation caused generally decreased responding to another CS that was paired with the same US. There are several possible explanations for this finding. The first is that extinction forms a new CS-no US memory. The present results provide little support for the hypothesis that extinction acts to form a new memory in US-retrieval extinction because the “CS-no US” memory produced by extinction would be specific to the extinguished CS and does not generalize to other cues without extinction (25). Additionally, the return of fear in spontaneous recovery and reinstatement is generally thought to be attributable to the unmasking of the original CS-US memory from suppression by the CS-no US memory (5), but the present results found no spontaneous recovery or reinstatement after US retrieval-extinction. Therefore, our results suggest that the US-retrieval extinction procedure causes generalization through some other process (4, 26, 27). The second explanation is that extinction causes US devaluation (27–29). According to this explanation, after extinction, the CS-US association remains intact, but the CS activates a weaker US representation that elicits a weaker fear response (29, 30). This explanation was supported by the finding that explicitly giving habituation training for the US also produced a decrease in conditioned fear responding, similar to that seen with CS extinction (27, 28). However, the reduction of responding after extinction was also sensitive to reinstatement if extinction in our procedure was attributable to US devaluation because the reinstatement shock would remind the subject of the strong aversive value of the US (29, 31). Thus, the US-devaluation explanation for extinction does not fit with our US-retrieval extinction procedure. A third explanation is that extinction during US-triggered reconsolidation induces generalization by disrupting sensory discrimination (30). Subjects that underwent US-retrieval extinction may be overgeneralizing between the different cues, such that extinction to one cue generalizes to another. However, unclear is why this would occur when the different cues predict the same US but not when the different cues predict the distinct USs.
We suggest that US-retrieval extinction may diminish the fear response by erasing or disassociating the original CS-US association to prevent the return of fear. Although extinction does not appear to cause this sort of memory disruption in most cases, this is consistent with previous studies, in which a pharmacological manipulation after US retrieval disrupted US-specific fear memories in rats (32, 33), and CS retrieval was shown to allow extinction to disrupt fear memory in humans and rodents (15, 16). The present results also showed that US-retrieval extinction is effective for remote fear memory. Moreover, our molecular results showed that US retrieval induced stronger alterations in reconsolidation-related molecules than CS retrieval (34, 35), suggesting that US retrieval induced more unstable reconsolidation. Although the strong US reactivation, that retards the extinction, may also induce even stronger biochemical changes than the weak US reactivation, some specific mechanism, e.g. the strong intrinsic fear arousal induced by the strong US, may underlie the prevention of extinction by stronger US reactivation (36). Although US retrieval-extinction causes a generalization effect, it only affected the reactivated US-related memories. CS retrieval destabilized the memory traces specific to the CS, while US retrieval destabilized the memory traces sensory specific to the US. Thus, if one US is associated with several CSs, US retrieval may have a greater influence on memory system and destabilize multiple memory traces (37). When several CSs are conditioned to one US, several separate CS-US associations are established, which makes the CS triggered reconsolidation specific to the reactivated CS-US association (4). Consistent with this, we found that the molecular alterations induced by US retrieval were stronger than CS retrieval. Although our findings indicated that US retrieval-extinction may share similar mechanisms with CS retrieval–extinction procedures, whether the mechanism that extinguishes one memory trace would influence the whole memory is unknown. We speculate that it may involve a neural circuit-composed of the medial prefrontal cortex, the nucleus reuniens (NR), and the hippocampus-that has been demonstrated to control fear memory generalization. Other factors could also influence the generalization effect of US-retrieval extinction, such as the strength or age of the memories, the duration of the reminder (38–40), and the interval between acquisitions of several CS-US associations. The precise mechanism needs further studies. Clinically, the selectivity of reconsolidation processes has significant value. Importantly, US-retrieval extinction based therapy for human anxiety disorders should reduce fear responses and not cause a total loss of fear responses to all fear-inducing cues. Furthermore, we found that exposure to a weak US before extinction training eliminated the fear response, whereas exposure to a US with similar intensity as that in the conditioning phase retarded the extinction of fear. Thus, if subjects were exposed to a real trauma that is nearly same as the one they experienced before, the fear response might not be successfully extinguished. We consider that a trauma which is which is similar to, but milder than, the original one may be helpful and feasible in clinic, although the precise intensity of US and precise methods require further investigation.
In conclusion, the present study demonstrated a new behavioral procedure to elicit generalized decreases in fear, with effects that lasted for at least 6 months. The memories of aversive events are often linked to multiple cues, and these cues may act as reminders of the aversive events. Because inhibiting responding to all previously reinforced cues using CS retrieval-extinction-based therapies to eliminate all unhealthy fear associations is impractical, the US retrieval-extinction behavioral procedure may open new avenues for preventing the return of fear. Future studies should determine the precise brain systems and cellular and molecular mechanisms of this phenomenon.
Supplementary Material
Acknowledgments
This work was supported in part by the National Basic Research Program of China (no. 2009CB522000) and Natural Science Foundation of China (no. 31230033, 91132716, and 31070958). The preparation of this paper was supported in part by the Intramural Research Program of the National Institutes of Health/National Institute on Drug Abuse. We thank Dr. Yavin Shaham and Shiqiu Meng for their helpful comments on the manuscript.
Footnotes
Financial Disclosures
The authors declare that they do not have any conflicts of interest related to the data presented in this manuscript.
References
- 1.Gerardi M, Cukor J, Difede J, Rizzo A, Rothbaum BO. Virtual reality exposure therapy for post-traumatic stress disorder and other anxiety disorders. Curr Psychiatry Rep. 2010;12:298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- 2.McLean CP, Foa EB. Prolonged exposure therapy for post-traumatic stress disorder: a review of evidence and dissemination. Expert Rev Neurother. 2011;11:1151–1163. doi: 10.1586/ern.11.94. [DOI] [PubMed] [Google Scholar]
- 3.Eysenck HJ. Behavior therapy and the conditioning model of neurosis. International Journal of Psychology. 1981;16:343–370. [Google Scholar]
- 4.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 6.Rachman SJ. The return of fear: review and prospect. Clinical Psychology Review. 1989;9:147–168. [Google Scholar]
- 7.Dudai Y. Reconsolidation: the advantage of being refocused. Curr Opin Neurobiol. 2006;16:174–178. doi: 10.1016/j.conb.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Lee JL. Reconsolidation: maintaining memory relevance. Trends Neurosci. 2009;32:413–420. doi: 10.1016/j.tins.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- 10.Nader K, Hardt O. A single standard for memory: the case for reconsolidation. Nat Rev Neurosci. 2009;10:224–234. doi: 10.1038/nrn2590. [DOI] [PubMed] [Google Scholar]
- 11.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 12.Nikzad S, Vafaei AA, Rashidy-Pour A, Haghighi S. Systemic and intrahippocampal administrations of the glucocorticoid receptor antagonist RU38486 impairs fear memory reconsolidation in rats. Stress. 2011;14:459–464. doi: 10.3109/10253890.2010.548171. [DOI] [PubMed] [Google Scholar]
- 13.Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flavell CR, Barber DJ, Lee JL. Behavioural memory reconsolidation of food and fear memories. Nat Commun. 2011;2:504. doi: 10.1038/ncomms1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schiller D, Raio CM, Phelps EA. Extinction training during the reconsolidation window prevents recovery of fear. J Vis Exp. 2012:e3893. doi: 10.3791/3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, et al. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Debiec J, Diaz-Mataix L, Bush DE, Doyere V, Ledoux JE. The amygdala encodes specific sensory features of an aversive reinforcer. Nat Neurosci. 2010;13:536–537. doi: 10.1038/nn.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubin FD, Sweatt JD. The IkappaB kinase regulates chromatin structure during reconsolidation of conditioned fear memories. Neuron. 2007;55:942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, et al. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nat Neurosci. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- 22.Gafford GM, Parsons RG, Helmstetter FJ. Memory accuracy predicts hippocampal mTOR pathway activation following retrieval of contextual fear memory. Hippocampus. 2013;23:842–847. doi: 10.1002/hipo.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diaz-Mataix L, Debiec J, Ledoux JE, Doyere V. Sensory-specific associations stored in the lateral amygdala allow for selective alteration of fear memories. J Neurosci. 2011;31:9538–9543. doi: 10.1523/JNEUROSCI.5808-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19:571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 26.Schiller D, Cain CK, Curley NG, Schwartz JS, Stern SA, Ledoux JE, et al. Evidence for recovery of fear following immediate extinction in rats and humans. Learn Mem. 2008;15:394–402. doi: 10.1101/lm.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storsve AB, McNally GP, Richardson R. US habituation, like CS extinction, produces a decrement in conditioned fear responding that is NMDA dependent and subject to renewal and reinstatement. Neurobiol Learn Mem. 2010;93:463–471. doi: 10.1016/j.nlm.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Rescorla RA. Effect of US habituation following conditioning. J Comp Physiol Psychol. 1973;82:137–143. doi: 10.1037/h0033815. [DOI] [PubMed] [Google Scholar]
- 29.Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J Exp Psychol Anim Behav Process. 1975;1:88–96. [PubMed] [Google Scholar]
- 30.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: effects on reacquisition and generalized extinction. Biol Psychiatry. 2005;57:841–847. doi: 10.1016/j.biopsych.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 31.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 32.Lai CS, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 33.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of of reinforcement and nonreinforcement. In: Prokasy AH, editor. Classical Conditioning II: Current Research and Theory. New York: Appleton-Century-Croft; 1972. pp. 64–99. [Google Scholar]
- 34.Lee JL, Hynds RE. Divergent cellular pathways of hippocampal memory consolidation and reconsolidation. Hippocampus. 2013;23:233–244. doi: 10.1002/hipo.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 36.Maren S, Chang CH. Recent fear is resistant to extinction. Proc Natl Acad Sci U S A. 2006;103:18020–18025. doi: 10.1073/pnas.0608398103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- 38.Pitman RK, Milad MR, Igoe SA, Vangel MG, Orr SP, Tsareva A, et al. Systemic mifepristone blocks reconsolidation of cue-conditioned fear; propranolol prevents this effect. Behav Neurosci. 2011 doi: 10.1037/a0024364. [DOI] [PubMed] [Google Scholar]
- 39.Costanzi M, Cannas S, Saraulli D, Rossi-Arnaud C, Cestari V. Extinction after retrieval: effects on the associative and nonassociative components of remote contextual fear memory. Learn Mem. 2011;18:508–518. doi: 10.1101/lm.2175811. [DOI] [PubMed] [Google Scholar]
- 40.Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.