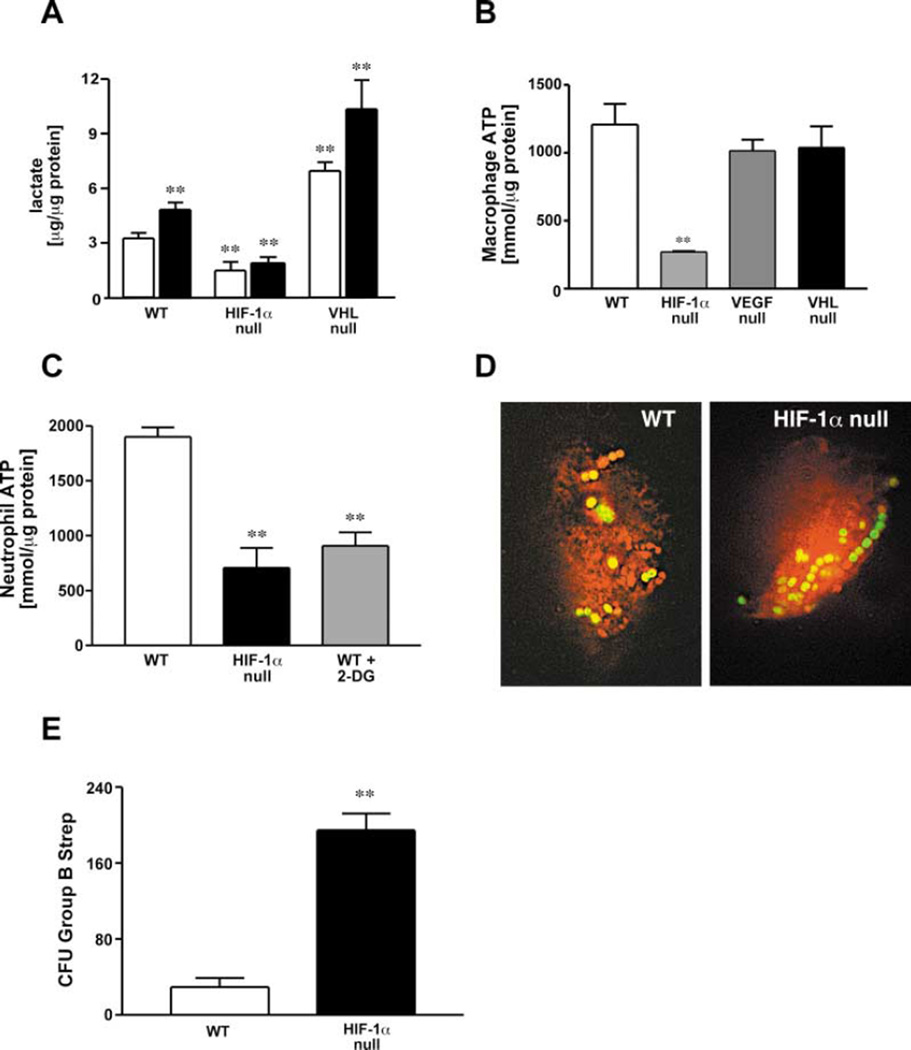
Figure 3. Glycolysis and Energy Generation in Myeloid Cells Are Severely Affected by the Loss of HIF-1α.
(A) Lactate concentrations in macrophage supernatant were quantified under either control conditions (open bars) or after the addition of LPS (closed bars). Values were normalized to total protein content.
(B) Peritoneal macrophages were isolated from WT, HIF-1α-, VEGF-, and VHL-LysM-CRE mice and cultured under ambient conditions for 24 hr. Cell lysates were harvested and intracellular ATP concentrations measured by means of a luciferase-based chemiluminescent assay. Values were normalized to total protein content.
(C) Peritoneal neutrophils were harvested and assayed for ATP synthesis. 2-deoxyglucose (500 mg/kg b.w.) was injected i.p. 60 min prior to harvest to block glycolysis in peritoneal exudate cells.
(D) Engulfment of viable bacteria was characterized by inoculating macrophages with GFP-expressing GBS for 2 hr. Deconvolution fluorescence microscopy was used for documentation.
(E) Bone marrow derived macrophages were inoculated with Group B streptococci (GBS) at a MOI of 2.5 and intracellular killing analyzed by determination of viable colony forming units in the macrophage lysates after washing and antibiotic treatment to remove nonengulfed bacteria.
Statistical analysis was performed using the unpaired Student’s t test, **p < 0.01.