Table 1.
The nature of the ligand and borane affect the distribution of borylated products and their derived alcohols.a
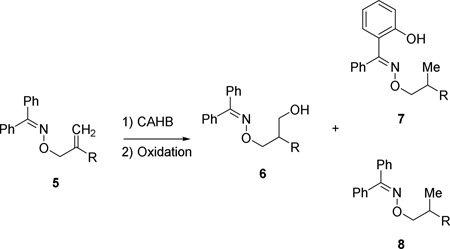 | ||||||
|---|---|---|---|---|---|---|
| entry | 5 R = | L | borane | 6 (er) | 7 | 8 |
| 1 | a Me | L1 | tmdBH | 6 (56:44) | 56 | 30 |
| 2 | a Me | L2 | tmdBH | 58 (67:34) | 31 | 10 |
| 3b | a Me | L2 | pinBH | 30 (56:44) | 16 | 24 |
| 4 | a Me | L2 | catBH | 63 (53:47) | 1 | 6 |
| 5 | b iBu | L2 | tmdBH | 52 (80:20) | 29 | 16 |
| 6 | b iBu | L2 | catBH | 16 (50:50) | 0 | 0 |
| 7 | c cC6H11 | L2 | tmdBH | 60 (70:30) | 11 | 28 |
| 8 | c cC6H11 | L2 | catBH | 52 (85:15) | 2 | 5 |
Reaction conditions: 2% [(L)2Rh(nbd)]BF4, 2 equiv. borane, THF, rt.
16% of the isomeric β-hydroxy isomer also formed.
