Abstract
Background
We have investigated β3-adrenoceptor agonist mediated brown adipose tissue (BAT) activation using 18F-FDG PET/CT in Zucker lean (ZL) and obese (ZF) rats.
Methods
18F-FDG was injected into ZL and ZF rats pretreated with saline or agonist CL316,243 for scans. 18F-FDG metabolic activity was computed as standard uptake values.
Results
CL316,243 in ZL activated BAT up to 4-fold compared to saline, while ZF BAT was only up by 2 fold. The decreased activation was consistent with lower β3-adrenoceptor levels in ZF rats.
Conclusions
The genetically modified ZL and ZF rats may provide a useful rat model to evaluate the significance of β3-adrenoceptor agonist-induced BAT activation in obesity.
Keywords: Obesity; β3-Adrenoceptor agonist; CL316,243; 18F-FDG; PET/CT
Introduction
β3 adrenergic receptor, or adrenoceptor induced activation of brown adipose tissue (BAT) is being explored as a potential therapeutic mechanism for obesity since BAT regulate the breakdown of lipids and glucose [1]. Activated BAT may thus have therapeutic potential to combat both diabetes and obesity with its ability to reduce plasma triglyceride levels [2]. CL316,243, a β3-adrenoceptor selective agonist, has been shown to enhance the uptake of 18F-FDG in BAT of normal rats [3] and mice [4,5]. More recently another β3-adrenoceptor agonist, mirabegron, has been shown to activate BAT in rats [6] and humans [7]. Thus, β3-adrenoceptor agonists enhance 18F -FDG uptake in BAT in vivo, signifying higher thermogenesis and a possible therapeutic role of these agonists for reducing diabetes and obesity.
Since BAT is activated in humans by β3-adrenoceptor agonist mirabegron [7] and the observation that repeat administration of β3-adrenoceptor agonist CL316,243 in rodents can induce white adipose tissue (WAT) browning [5], therapeutic potential of these drugs for weight reduction by enhancing thermogenesis in both rats and humans is plausible. Furthermore, therapeutic efficacy of these drugs may be evaluated by 18F-FDG PET in longitudinal studies [5]. The genetically modified Zucker obese (fa/fa) and lean (Fa/Fa) rat provides a useful rat model to evaluate the significance of β3-adrenoceptor agonist-induced BAT activation for weight reduction.
In order to evaluate this β3-adrenoceptor agonist induced thermogenesis effect in the obesity model, we used the obese Zucker (fa/fa) rat (ZF) and compared it to the lean Zucker (Fa/Fa) rat (ZL). Brain 18F -FDG PET studies in the ZF model have been reported to study the central effects of leptin-receptor deficiency [8,9]. However, there are no reports of 18F –FDG PET studies of BAT in the ZF rat. We report our preliminary findings of CL316,243 induced 18F -FDG uptake in BAT using PET/CT studies in this ZF rat model.
Methods
Radioactivity was counted using a CRC-R dose calibrator (Capintec, Inc., Ramsey, NJ, USA). An Inveon PET/CT scanner (Siemens Medical Solutions, Inc., Malvern, PA, USA) was used in docked mode for PET/CT scans. All images were analyzed using ASIPro VM and Inveon Research Workplace (Siemens Medical Solutions, Inc.). All animal studies were approved by the Institutional Animal Health Care and Use Committee of the University of California – Irvine. Adult male Zucker rats (ZL and ZF, n=2 each) purchased from Harlan Laboratories, Inc., Placentia, CA, USA) were housed under controlled temperature of 22°C±1°C. All rats were fasted for 24h before 18F-FDG administration. Either CL316,243 (2 mg/kg; Tocris Bioscience, Ellisville, MO, USA; [3]) or normal saline was administered 30 minutes before 18F-FDG administration. After 18F-FDG (15 to 22 MBq) administration intravenously, the rats were awake for 60 minutes. All saline and CL316,243-treated rats were placed in a supine position in a rat holder and anesthetized using 2% isoflurane and then maintained under anesthesia for upper-body PET and CT imaging (4 ZL scans and 4 ZF scans; all saline scans were carried out prior to CL316243 scans in order to avoid any residual drug effects). The rat holder was placed on the PET/CT bed, and all animals had a CT scan after the PET scan for attenuation correction and anatomical delineation of PET images. For quantitative PET analysis, regions of interest (ROIs) were drawn on respective PET images using ASIPro and IRW. The ROIs were delineated visually by contouring the 18F-FDG activity that was clearly above the normal background activity. The magnitude of BAT 18F-FDG activation was expressed as standard uptake value (SUV) which was defined as the average 18F-FDG activity in each volume of interest [VOI] (KBq/cm3) divided by the injected dose (MBq) times the body weight of each animal (Kg). The SUVs were thus expressed in the units of (KBq/cm3/(MBq/Kg)). Hounsfield units of the BAT areas were computed from the CT scans. Statistical differences between groups were determined using students t test. A p value of <0.05 was considered to indicate statistical significance.
Results
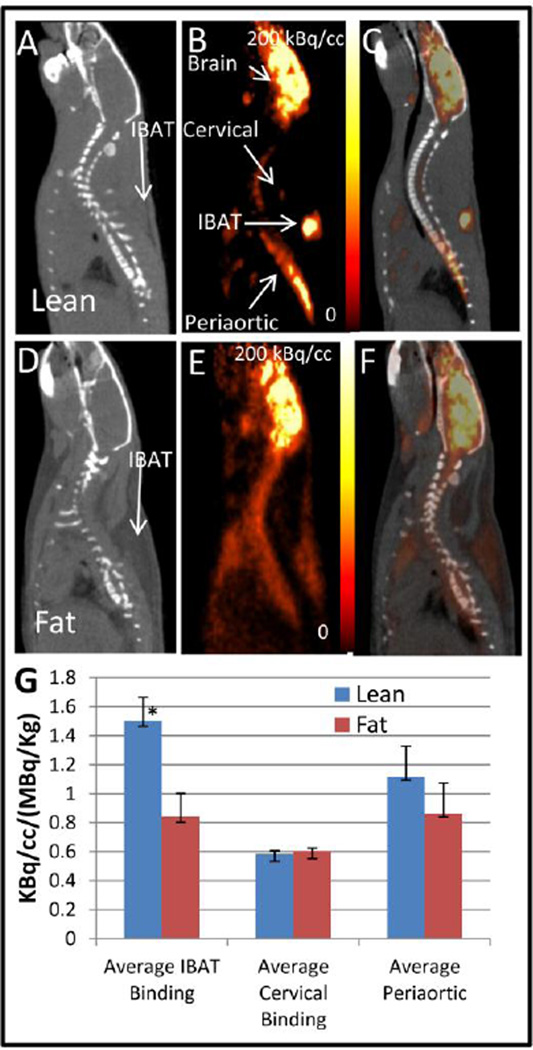
Fasted ZL rats exhibited highest 18F-FDG uptake in the brain followed by the various BAT regions, interscapular BAT (IBAT), cervical BAT and periaortic BAT (Fig-1 A–C). The IBAT was visualized in the CT image surrounded by white adipose tissue (WAT) (Fig-1A) with CT measures of −68±6 HU for IBAT and −181±2 HU for WAT (Table-1). These values for IBAT and WAT were similar to that measured in the Sprague-Dawley rats [10]. The CT of the ZF rats (Fig-1D) had a distinctly lower density for IBAT (−253±10 HU; p <0.05 compared to ZL) which was comparable to WAT of the ZL rats. The SUV values of 18F-FDG uptake in the BAT regions was 1.5±0.2 in IBAT, 1.1±0.2 in periaortic and 0.6±0.02 in cervical and is consistent with those found in normal Sprague Dawley rats [3]. The uptake of 18F-FDG in the IBAT region of the ZF was diffused as seen in Fig-1E with an SUV of 0.8±0.04 which is approx. 56% of that measured in the ZL rat (Fig-1G).
Figure 1. Uptake of 18F-FDG in saline pretreated rats.
CT (A), PET (B) and coregistered PET-CT (C) image of ZL rats pretreated with saline showing uptake of 18F-FDG in the IBAT, cervical and periaortic area. CT (D), PET (E) and coregistered PET-CT (F) image of ZF rats pretreated with saline showing uptake of 18F-FDG in the IBAT, cervical and periaortic area. (G) Graph showing a comparison in uptake of 18F-FDG in lean versus fat rats pretreated with saline for the IBAT (p <0.05, asterisk), cervical and periaortic area.
Table-1.
Zucker lean Fa/Fa and fat fa/fa PET and CT Measures
| Measure | PET (KBq/cc/(MBq/Kg)) | CT (Hounsfield) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | IBAT | Cervical | Periaortic | Brain | BAT | WAT | ||||||
| Zucker Rat |
Lean | Fat | Lean | Fat | Lean | Fat | Lean | Fat | Lean | Fat | Lean | Fat |
| Saline | 1.50±0.15* | 0.84±0.04 | 0.58±0.02 | 0.60±0.05 | 1.11±0.21 | 0.86±0.02 | 2.31±0.18 | 1.98±0.31 | −68±5.7* | −253±10 | −181±2.3 | −238±24 |
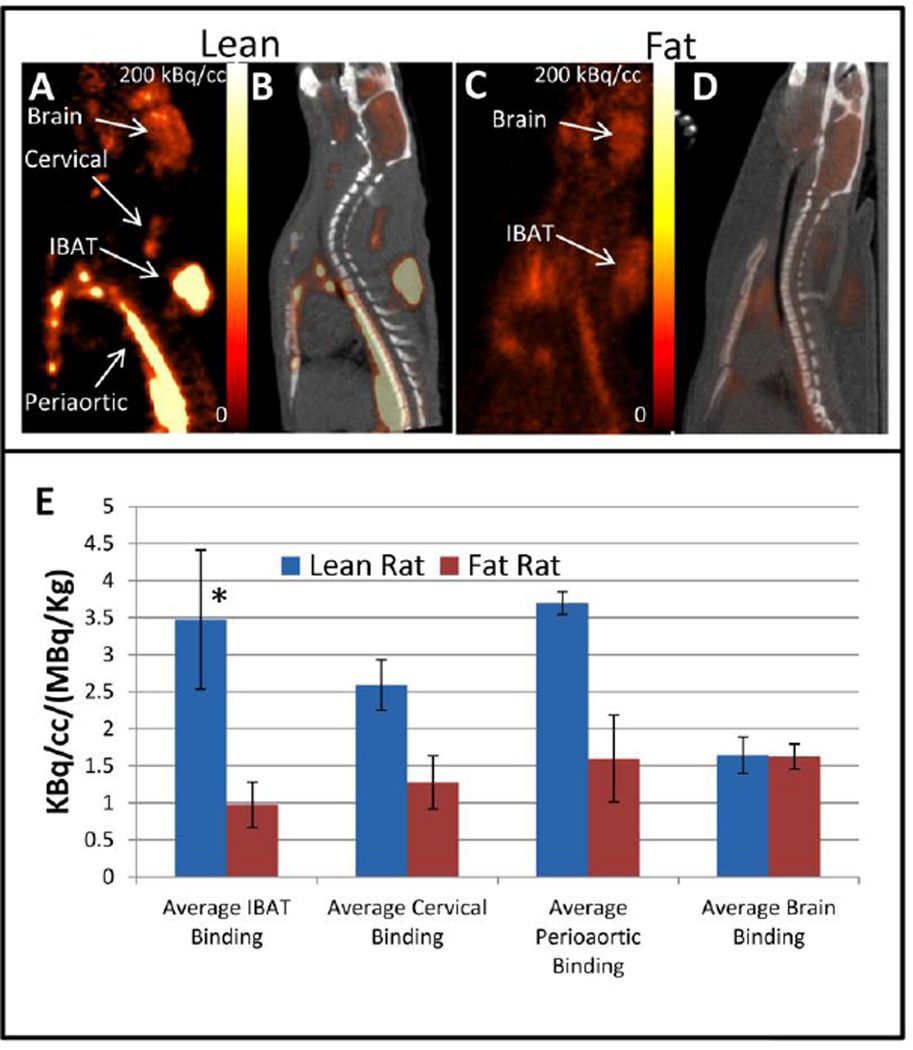
| CL316, 243 | 3.47±0.94* | 0.97±0.30 | 2.59±0.34 | 1.27±0.35 | 3.69±0.15 | 1.59±0.58 | 1.64±0.24 | 1.62±0.17 | −115±16* | −276±8 | −258±9.8 | −314±8 |
PET and CT data acquired after 18F-FDG injection in ZL (n=2) and ZF (n=2) rats first using saline and on a different day CL316,243 (2 mg/kg).
Lean Fa/Fa versus fat fa/fa (p <0.05).
Pretreatment with CL 316,243 was well tolerated in all the animals. The ZL rats showed significant increase of 18F-FDG in all the BAT regions (Fig-2A). A two- to four-fold increase occurred in all the BAT regions as a result of activation by the β3 adrenoceptor agonist in the lean rats (Table-1). The increase in the Zucker rats is consistent with our findings in the Sprague Dawley rats, although the magnitude of the increase is smaller [3]. In the ZF rat, while there was an increase in 18F-FDG uptake in the BAT regions, IBAT showed only a marginal increase of 15% (Fig-2C; Table-1). Cervical and periaortic BAT showed greater increases after treatment with CL 316,243, but were still lower than that measured for the ZL rats. No significant change in opacity was observed in the CT scans in both the lean and fat rats after a single dose of CL 316,243 compared to the saline treated scans (Table-1).
Figure 2. Uptake of 18F-FDG in CL316,243 pretreated rats.
PET (A,C) and coregistered PET-CT (B,D) image of ZF and ZL rats pretreated with CL316,243, respectively showing uptake of 18F-FDG in the IBAT, cervical and periaortic area. (E) Graph showing a comparison in uptake of 18F-FDG in lean versus fat rats pretreated with CL316,243 for the IBAT (p <0.05, asterisk), cervical and periaortic area.
In both the ZL and ZF rats, under baseline conditions with saline preinjections, brain uptake of 18F-FDG was higher than BAT regions (Table-1). The ZF rat showed an approx. 85% 18F-FDG uptake of that found in the lean rat. With the CL 316,243 preinjections, the brain uptake of both the lean and fat were similar (Fig-2E). CL 316,243 induced a greater decrease in 18F-FDG brain uptake in the ZL rats (70% of saline), similar to our previous findings with Sprague-Dawley rats [3]. The 18F-FDG brain uptake of the ZF rat on the other hand decreased to 82%, compared to saline pretreated rats.
Discussion
Our findings show a reduced thermogenic effect of BAT in the ZF rat model compared to the ZL model. There was less IBAT activity observed in the ZF rat, which may be due to the lower levels of β3 adrenoceptors present in the ZF rats compared to the ZL rats [11]. Various abnormalities such as central metabolism regulation in the tissue and neuroendocrine metabolism may also contribute to BAT thermogenesis impairment [11]. Adrenalectomy in obese rats, according to another previous study, was able to restore normal BAT thermogenesis, with abnormal thermogenesis initially due to anomalous responses to glucocorticoids and sympathetic stimuli [12]. It was further suggested that an adrenal factor, most plausibly insulin, plays a role in down regulation of β3 adrenoceptor mRNA levels; therefore, with adrenalectomy, reverse effects are seen in the ZF rat. In this leptin-receptor deficient fa/fa model, the coupling of the β3 adrenoceptors with the G-protein is reportedly reduced in white adipocytes as well [13]. Thus, in saline-treated condition, the significantly lower IBAT activity in the ZF rats is most likely related to the down regulation of β3 adrenoceptors.
Consistent with our previous observations with Sprague-Dawley rats [3], quantifiable BAT activation was measured in the ZL rat model in the presence of the agonist CL316,243 due to activation of β3 adrenoceptors. Despite the lower β3-adrenoceptor levels and reduced G-protein coupling in the ZF rat model, the agonist CL316,243 had some measureable effects on BAT. Although IBAT, cervical and periaortic BAT had increased 18F-FDG uptake, the change was not significant. The CT scans showed a significantly low opacity in ZF compared to ZL, suggesting low abundance of brown adipocytes in the IBAT region, consistent with the lower 18F-FDG uptake. Although no significant effect of an acute dose of CL316,243 in the CT scans was noted in either ZL or ZF (Table-1), repeat injection studies of the agonist CL316,243 may be useful to evaluate potential conversion of white adipocytes to beige or brown adipocytes as previously reported [5]. Difficulties in precise delineation of the BAT regions (IBAT, cervical and periaortic) and WAT using the CT scans in the ZF rats contributed to the lower significance of some of the PET and CT measures.
There is renewed focus on the development of therapeutics to restore leptin receptor function in order to address human obesity [14]. Our preliminary findings from the leptin-receptor deficient fa/fa rat model demonstrate that the residual β3 adrenoceptors conserved in this rat model are functional with respect to enhancing metabolic activity. The sensitivity to detect this enhanced BAT metabolic activity via the β3-adrenoceptors of 18F-FDG PET/CT method may therefore be useful in the development and evaluation of novel treatment strategies targeting the leptin receptor pathways.
Acknowledgements
We like to thank NIH grants RC1 DK087352 and R21 DK092917 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown fat in humans: Consensus points and experimental guidelines. Cell Metab. 2014;20:408–415. doi: 10.1016/j.cmet.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Revs Endocrinology. 2014;10:24–36. doi: 10.1038/nrendo.2013.204. [DOI] [PubMed] [Google Scholar]
- 3.Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J. Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and β3-adrenergic receptor activation. EJNMMI Res. 2011;1:30. doi: 10.1186/2191-219X-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mirbolooki MR, Upadhyay SK, Constantinescu C, Pan ML, Mukherjee J. Adrenergic pathway activation enhances brown adipose tissue metabolism: A 18F-FDG PET/CT study in mice. Nucl Med Biol. 2014;41:10–16. doi: 10.1016/j.nucmedbio.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JW, Jung K, Lee JH, Quach CH, Moon SH, Cho YS, Lee KH. 18F-FDG PET/CT Monitoring of β3 Agonist-Stimulated Brown Adipocyte Recruitment in White Adipose Tissue. J Nucl Med. 2015;56:153–158. doi: 10.2967/jnumed.114.147603. [DOI] [PubMed] [Google Scholar]
- 6.Mirbolooki RM, Schade KN, Constantinescu CC, Pan M-L, Mukherjee J. Enhancement of 18F-fluorodeoxyglucose metabolism in rat brain frontal cortex using a β3 adrenoceptor agonist. Synapse. 2015;69:96–98. doi: 10.1002/syn.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypess AM, Weiner LS, Roberts-Toler C, Elia EF, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, Kolodny GM. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–38. doi: 10.1016/j.cmet.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtanen KA, Haaparanta M, Gronroos T, Bergman J, Solin O, Rouru J, Nuutila P, Huupponen R. 2-[18F]fluoro-2-deoxy-D-glucose combined with microdialysis scan be used for the comparison of tissue glucose metabolism in obese and lean rats. Diabetes, Obesity and Metab. 2002;4:60–68. doi: 10.1046/j.1463-1326.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 9.Liistro T, Guiducci L, Burchielli S, Panetta D, Belcari N, Pardini S, Del Guerra A, Salvadori PA, Iozzo P. Brain glucose overexposure and lack of acute metabolic flexibility in obesity and type 2 diabetes: a PET-[18F]FDG study in Zucker and ZDF rats. J. Cereb Blood Flow Metab. 2010;30:895–899. doi: 10.1038/jcbfm.2010.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirbolooki MR, Constantinescu C, Pan ML, Mukherjee J. Targeting presynaptic norepinephrine transporter in brown adipose tissue: A novel imaging approach and potential treatment for diabetes and obesity. Synapse. 2013;67:79–93. doi: 10.1002/syn.21617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levin BE, Finnegan MB, Marquet E, Sullivan AC. Defective brown adipose oxygen consumption in obese Zucker rats. Am. J. Physiol. 1984;247:E94–E100. doi: 10.1152/ajpendo.1984.247.1.E94. [DOI] [PubMed] [Google Scholar]
- 12.Onai T, Kilroy G, York DA, Bray GA. Regulation of β3-adrenergic receptor mRNA by sympathetic nerves and glucocorticoids in BAT of Zucker obese rats. Am. J. Physiol. 1995;269:R519–R526. doi: 10.1152/ajpregu.1995.269.3.R519. [DOI] [PubMed] [Google Scholar]
- 13.Mory G, Wiel M, Adli H, Diot-Dupuy F, Ferre P, Bazin R. Impaired β-adrenergic signaling pathway in white adipocytes of suckling fa/fa Zucker rats: a defect in receptor coupling. Internat. J. Obesity. 2001;25:1592–1598. doi: 10.1038/sj.ijo.0801811. [DOI] [PubMed] [Google Scholar]
- 14.Roujeau C, Jockers R, Dam J. New pharmacological perspectives for the leptin receptor in the treatment of obesity. Front Endocrinol (Lausanne) 2014;5:167. doi: 10.3389/fendo.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]