Abstract
Objective
To estimate the prevalence and predictors of moderate/severe poor sleep in relation to the final menstrual period (FMP) of mid-life women.
Methods
Annual assessments were conducted in a population-based cohort of 255 women. All were premenopausal at cohort enrollment and reached natural menopause during the 16-year follow-up. The outcome measure was the severity of poor sleep, as reported by the participants in annual interviews for 16 years and evaluated in relation to the FMP.
Results
The annual prevalence of moderate/severe poor sleep largely ranged from about 28% to 35%, with no significant differences in any year relative to the FMP for the sample overall. When sleep status was stratified at the premenopausal baseline, the premenopausal sleep status strongly predicted poor sleep around the FMP. Women with moderate/severe poor sleep when premenopausal were approximately 3 ½ times more likely to have moderate/severe poor sleep around menopause compared to those with no poor sleep at baseline in adjusted analysis (OR 3.58, 95% CI: 2.50-5.11, P<0.0001), while mild poor sleepers premenopause were approximately 1 ½ times more likely to have moderate/severe poor sleep around menopause (OR 1.57, 95% CI: 0.99-2.47, P=0.053). There was no significant association between poor sleep and time relative to the FMP among women who had no poor sleep at the premenopausal baseline. Hot flashes were significantly associated with poor sleep (OR 1.79, 95% CI: 1.44-2.21, P<0.0001 in adjusted analysis), but had no interaction with baseline sleep severity (interaction P=0.25), indicating that hot flashes contributed to poor sleep regardless of baseline sleep status.
Conclusion
The findings showed a high prevalence of moderate/severe poor sleep in mid-life women, with only a small “at risk” subgroup having a significant increase in poor sleep in relation to the FMP. Sleep status at the premenopausal baseline and concurrent hot flashes strongly and consistently predicted poor sleep in the menopause transition. Overall, poor sleep did not increase around the FMP and frequently occurred in the absence of hot flashes, indicating that sleep difficulties in the menopause transition in generally healthy women were not simply associated with ovarian decline.
Keywords: menopause, sleep, insomnia, hot flashes, longitudinal
Introduction
One of the most common complaints of mid-life women is poor sleep. About 30% of adults have one or more symptoms of insomnia, according to the American Academy of Sleep Medicine and population-based studies.1, 2 Poor sleep is more prevalent in women, with gender differences emerging around menarche and further increasing in mid-life.3-5 Mid-life sleep difficulties are often attributed to symptoms of menopause. Poor sleep is clearly associated with hot flashes, the cardinal symptom of menopause,6. 7 but evidence that the biological decline in ovarian function has direct effects on sleep is limited and controversial.8. 9 Furthermore, a time course of poor sleep relative to the final menstrual period, which is the primary marker of ovarian senescence, has not been identified.
We previously evaluated poor sleep in the early years of the Penn Ovarian Aging cohort, when the participants were premenopausal and had regular menstrual cycles.10, 11 At that time, the prevalence of moderate/severe poor sleep was approximately 17%, but the subsequent time course of poor sleep in relation to the final menstrual period could not be determined. Data are now available to estimate the pattern of poor sleep relative to natural menopause. We hypothesized that moderate/severe poor sleep was associated with the biological changes of ovarian aging with a pattern of increasing poor sleep in the years leading to the final menstrual period (FMP), a peak around the FMP and a decrease in the years after menopause. We also hypothesized that the severity of poor sleep at the premenopausal baseline predicted poor sleep across the menopause transition. Additional objectives were to evaluate possible risk factors for poor sleep, including hot flashes, anxiety, depression, perceived stress, race, age at menopause, body mass index and smoking. Longitudinal associations between reproductive hormone levels as markers of the menopause transition (estradiol, follicle stimulating hormone (FSH), inhibin b) and poor sleep were also evaluated.
Methods
Study participants
The study evaluated 255 women in the Penn Ovarian Aging Study (POAS) cohort who reached natural menopause during a 16-year follow-up period (1996-2012). We included only the participants who reached natural menopause in order to address our primary aim of estimating the risk of poor sleep in relation to the FMP. Comparisons of study variables at baseline between the sample and the remainder of the cohort who were not observed to reach natural menopause during the follow-up period (n=181) showed no significant differences in the study variables, with the exception of age, which was older in the study group at baseline (42.2 vs 40.4y, P<0.001).
The original cohort of 436 women was randomly identified by telephone digit dialing in Philadelphia County, PA, using stratified sampling to obtain equal numbers of African American and white women as previously described.10 At enrollment, all women were premenopausal with regular menstrual cycles of 22-35 days for the previous three cycles, ages 35-48 years, had an intact uterus and at least one ovary. Exclusion criteria at enrollment included current use of any hormonal or psychotropic medications, alcohol or drug abuse, major psychiatric disorder in the past year, pregnancy or breast feeding, uncontrolled hypertension, and serious health problems known to compromise ovarian function. The Institutional Review Board of the University of Pennsylvania approved the study, and all participants provided written informed consent.
Study design
After cohort enrollment, follow-up assessments were conducted for 16 years at intervals of approximately 9 months and then annually. Study data were collected at two in-home visits, which were timed to the early follicular phase of the menstrual cycle (days 2-6) in two consecutive menstrual cycles, or approximately one month apart in non-cycling women for 14 assessment periods. Assessments 15-16 were conducted by telephone interview. The study was described to participants as a general women's health study. Trained research interviewers obtained menstrual dates, structured interview data on overall health, blood samples for hormone assays, and anthropometric measures. Participants completed a set of validated self-report measures to assess health and other behavioral measures of the study at each assessment period.
Study variables
The primary outcome variable was moderate/severe poor sleep, which was included in a validated menopausal symptom questionnaire that was administered at enrollment and at each of 16 years of follow-up.12 The interviewer asked “ Have you experienced trouble sleeping in the past month?” Further queries determined the frequency and severity (rated mild=1; moderate=2; severe=3) of poor sleep. After inspection of the response distribution for the severity of poor sleep, a 3-level variable was created for moderate/severe, mild and no poor sleep. Validity was evaluated by factor analysis conducted in the cohort, which indicated a high correlation of the sleep item with the sleep quality factor score derived from the St. Mary's Sleep Questionnaire (r=0.83).11 The sleep item was previously used as the primary outcome measure in studies in the cohort,10 supported by data showing that sleep quality was a better marker than sleep quantity for assessing general health and debilitating feelings such as depression, fatigue, tension and stress.13
Time in years, from 11 or more years before to 11 or more years after the FMP, was evaluated in relation to the FMP, which was identified after 12 or more months of no menstrual bleeding and designated as Time 0 for each participant. This allowed longitudinal evaluation of within-woman changes in poor sleep each year before the FMP (up to, but not including, Time 0) and each year following the FMP.
Covariate selections were based on previously identified associations with poor sleep and the aims of this study. The variables included age, race (self-reported as African American or white), body mass index (kg/m2 >=30, <30), current smoking (yes, no), alcohol use >=1/week (yes, no), currently employed, (yes, no), education (>HS, <=HS) and history of depression (yes, no) as identified at cohort enrollment by medical history interview or the Primary Care Evaluation of Mental Disorders interview.14 Anxiety, depressed mood and perceived stress were assessed at each assessment period using validated self-report questionnaires: the Zung Anxiety Index,15 the Center for Epidemiologic Studies Depression Scale (CES-D),16 and the Perceived Stress Scale (PSS).17 Each scale provided a continuous total score, with higher scores indicating more symptoms. The severity of hot flashes was reported by the participants at each assessment period using the validated menopausal symptom list embedded in the structured interview questionnaire.12 At each follow-up, the interviewer asked whether hot flashes/night sweats occurred in the past month, whether they occurred in the past year, and the severity of the hot flashes/night sweats (rated 0, none; 1, mild; 2, moderate; 3, severe). The response distributions for severity of hot flashes/night sweats were inspected and a dichotomous variable termed hot flashes (moderate/severe, mild/none) was used in analysis.
Blood samples were collected at each study visit (providing a possible maximum of 28 samples per woman), centrifuged and frozen in aliquots at -80C. Assays were conducted in batches that included four visits per participant to reduce the within-woman variability resulting from assay conditions. Assays of estradiol and follicle stimulating hormone (FSH) were conducted in the Translational Research Center of the University of Pennsylvania utilizing Coat-A-Count commercial kits (Siemens, Deerfield, IL). All assays were performed in duplicate and repeated if values differed by greater than 15%. Interassay and intra assay coefficients of variation were less than 5%. Dimeric inhibin b was assayed at Periods 11-14 using enzyme-linked immunosorbent kits (Diagnostic Systems, Webster, TX). The intra- and interassay coefficients of variation were 3.5% to 4.6% and 6.3% to 7.6%, respectively. The lower limit of detection was 15 pg/mL. Dimeric inhibin b at Periods 1-10 was measured from serum in the laboratory of Patrick Sluss, PhD, at the Massachusetts General Hospital (Boston, MA), using a sensitive 2-site, non isotopic immunoassay (Oxford BioInnovation, London, UK). The intra assay and interassay coefficients of variation were less than 8% and less than 20%, respectively, for concentrations of 50-500 pg/mL. The lower limit of detection was 15 pg/mL.
Statistical analysis
Longitudinal evaluations of the 255 participants provided 3,399 responses to the item assessing poor sleep. The prevalence of moderate/severe poor sleep was calculated for each year before and after the final menstrual period (FMP) and compared to prevalence at the final menstrual period (Time 0). Generalized linear mixed effects regression models for repeated measures used all available data from each participant to estimate bivariable and multivariable associations with moderate/severe poor sleep. All models utilized generalized estimating equations (GEE) variance estimates for the statistical tests on the regression coefficients to adjust for repeated observations from each participant in a robust fashion.18 The models were adjusted for time, which was defined in three segments relative to the FMP: 1) premenopause: from 11 or more years to, but not including, the FMP; 2) FMP up to 3 years post FMP; 3) late postmenopause: >=3 years post FMP. These cut points were previously identified based on data in the cohort19 and were consistent with the cut points suggested for early and late postmenopause in the revised STRAW-10 staging.20 A secondary cut point at 6 years after the FMP was evaluated with similar results.
Covariates were defined a priori and added singly to the model with time and the interaction with time. All time-varying covariates were treated as such in modeling. Hypothesized interactions with time were evaluated for severity of poor sleep at baseline, hot flashes, and mood variables. Covariates that were associated with moderate/severe poor sleep at P<=0.20 were included in multivariable models to determine their independent contributions to the outcome of moderate/severe poor sleep. Inclusion in the final multivariable models was guided by whether each variable remained statistically significant at P<=0.05 or modified other significant associations by >=15%. Race and age at FMP were not significant but were retained in the final models due to the study design. Hormone levels were modeled using natural log transformations to reduce the influence of large values. The subject mean of the two hormone measures obtained at each assessment period was used in analysis. Odds ratio estimates for the hormones are presented per 1 unit (standard deviation) change. Exogenous hormone use was an exclusion at enrollment in the cohort; hormone use during follow-up was low, and only the observations at times of use were omitted in analysis. Hysterectomy was an exclusion at enrollment; women who had a hysterectomy after enrollment and prior to natural menopause were excluded in this study. The few observations of pregnancy or breast feeding during the study were omitted from analysis only at times of occurrence; the data for these participants before and after these events were retained.
Statistical power calculations were computed using STATA version 13 (College Station, TX). Assumptions were based on data in the cohort and included a 30% prevalence of poor sleep for the unexposed or low risk group, an average of 13 repeated measures per participant, type I alpha error of 5%, and within woman correlation of 0.28. Given these assumptions, the current study had 80% power to detect an odds ratio of 1.5 or higher for risk factors with a low prevalence of 20%. For risk factors with prevalence of 30%, the detectible odds ratio was 1.39 and was even lower for risk factors with prevalence greater than 30%. All analyses were conducted using the SAS 9.3 statistical package (SAS, Inc., Cary, NC). Statistical tests were two-sided, with P<=0.05 considered significant.
Results
Sample description
The 255 participants who reached menopause provided a mean of 13.3 outcome reports per participant. At the premenopausal baseline, 28% of the participants reported moderate/severe poor sleep, 16% mild poor sleep and 56% no poor sleep. In the repeated assessments over the 16-year follow-up, 82.4% (210/255) ever reported moderate/severe poor sleep, 27 women (10.5%) reported only mild levels of poor sleep, and 18 women (7.1%) never reported poor sleep. The mean (SD) age was 42.16 (3.38) years at baseline and 51.47 (3.32) years at the FMP; 49% were African American and 51% were white (Table 1).
Table 1. Characteristics of the Sample at Baseline.
| Variable | Baseline, N=255 |
|---|---|
|
| |
| Mean (SD) | |
|
| |
| Age, y | 42.16 (3.38) |
|
| |
| Age at FMP, y | 51.47 (3.32) |
|
| |
| BMI, kg/m | 29.21 (7.75) |
|
| |
| Anxiety (Zung), mean(SD)1 | 34.59 (7.70) |
|
| |
| Perceived stress (PSS)2 | 21.00 (7.76) |
|
| |
| Depression (CES-D)3 | 14.76 (10.66) |
|
| |
| Physical health (SF12) | 50.11 (8.34) |
|
| |
| Estradiol, pg/mL | 43.67 (30.61) |
|
| |
| FSH, mlU/mL | 8.08 (3.74) |
| Inhibin b, ng/mL | 70.35 (42.21) |
|
| |
| N (%) | |
|
| |
| Poor sleep | |
| None | 143 (56.1) |
| Mild | 41 (16.1) |
| Moderate/severe | 71 (27.8) |
|
| |
| Hot flashes, yes | 94 (37.0) |
|
| |
| CES-D ≥16 | 100 (39.5) |
|
| |
| History of depression | 115 (45.1) |
|
| |
| Alcohol ≥1/wk | 26 (10.2) |
|
| |
| Current smoker | 102 (40.2) |
|
| |
| Employed | 215 (84.3) |
|
| |
| Education >HS | 144 (56.5) |
| ≤HS | 111 (43.5) |
|
| |
| Race | |
| African American | 124 (48.6) |
| White | 131 (51.4) |
Score categories by Zung are 20-35 (normal), 36-47 (moderate), 48-60 (high).
Mean score for community-based adult females is 25.6 (SD 8.2).
The standard cut point for high depressive symptoms is ≥ 16.
Prevalence of poor sleep around menopause
The annual prevalence of moderate/severe poor sleep primarily ranged from 28% to 35% and did not significantly differ relative to the FMP in any study year (P=0.72, Figure 1). At the FMP, the prevalence was 34%, and prevalence remained above 30% in each study year until late postmenopause, when poor sleep decreased slightly but not significantly.
Figure 1.
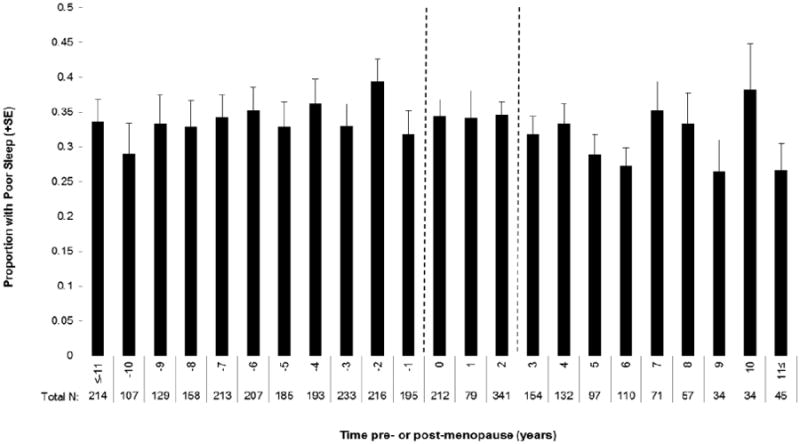
Proportion of women with moderate/severe poor sleep each year before and after the final menstrual period (Time 0). Dotted lines indicate the 3 time segments used in analysis: 1) from 11 or more years before, but not including, the FMP; 2) FMP up to 3 years post FMP; 3) >=3 years post FMP.
Stratification of sleep status at the premenopausal baseline to predict poor sleep
Stratification of sleep status reported by the participants at the premenopausal baseline strongly predicted poor sleep in the years around the FMP. Women with moderate/severe poor sleep at baseline (28% of the sample) were approximately 3 ½ times more likely to have moderate/severe poor sleep around the FMP compared to the women who had no poor sleep at baseline in adjusted analysis (OR 3.58, 95% CI: 2.50-5.11, P<0.0001); women with mild poor sleep at baseline (16% of the sample) were approximately 1 ½ times more likely to have moderate/severe poor sleep around the FMP (OR 1.57, 95% CI: 0.99-2.47, P=0.053) (Table 2).
Table 2. Unadjusted and Adjusted Associations of Study Variables with Subjective Poor Sleep.
| Adjusted for Time onlya | Final Multivariable Modelf | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P |
| Time | 0.454 | 0.013 | ||||
| 11y to FMP | 0.91 | 0.75-1.10 | 0.333 | 0.47 | 0.27-0.79 | 0.005 |
| FMP to 3y | Reference | Reference | ||||
| ≥3 after FMP | 0.88 | 0.72-1.09 | 0.265 | 0.52 | 0.25-1.09 | 0.082 |
|
| ||||||
| Baseline Severity of Poor Sleep | <0.0001 | <0.0001 | ||||
| None | Reference | Reference | ||||
| Mild | 1.53 | 0.99-2.38 | 0.056 | 1.57 | 0.99-2.47 | 0.053 |
| Mod/Severe | 2.98 | 1.83-4.85 | <0.0001 | 3.58 | 2.50-5.11 | <0.0001 |
|
| ||||||
| Hot flashes | <0.0001 | <0.0001 | ||||
| Mod/Severe | 1.78 | 1.50-2.11 | 1.79 | 1.44-2.21 | ||
|
| ||||||
| Anxiety (Zung scores)d | 1.10 | 1.08-1.12 | <0.0001 | 1.10 | 1.08-1.12 | <0.0001 |
|
| ||||||
| Stress (PSS)b,d | 0.032c | 0.016c | ||||
| 11y to FMP | 1.05 | 1.04-1.07 | <0.0001 | 1.02 | 1.00-1.04 | 0.030 |
| FMP to 3y | 1.02 | 1.00-1.04 | 0.057 | 0.98 | 0.96-1.01 | 0.206 |
| ≥3y after FMP | 1.06 | 1.03-1.09 | <0.001 | 1.02 | 0.99-1.06 | 0.184 |
|
| ||||||
| Age at FMP | 0.99 | 0.98-1.02 | 0.736 | 1.01 | 0.98-1.03 | 0.544 |
|
| ||||||
| Race | ||||||
| African Amer. | 1.08 | 0.78-1.49 | 0.636 | 0.80 | 0.58-1.01 | 0.169 |
| White | Reference | |||||
|
| ||||||
| Depression (CES-D) | ||||||
| >=16 | 2.13 | 1.74-2.61 | <0.0001 | |||
| < 16 | Reference | |||||
|
| ||||||
| History of Depression | 2.08 | 1.51-2.86 | <0.0001 | |||
|
| ||||||
| Employed | 0.79 | 0.51-1.22 | 0.288 | |||
|
| ||||||
| Education | ||||||
| ≤HS | 0.87 | 0.63-1.20 | 0.380 | |||
| > HS | Reference | |||||
|
| ||||||
| BMI | ||||||
| ≥30 kg/m2 | 1.08 | 0.84-1.40 | 0.543 | |||
| <30 kg/m2 | Reference | |||||
|
| ||||||
| Alcohol ≥ 1/wk | 0.96 | 0.73-1.27 | 0.776 | |||
|
| ||||||
| Current Smoker | 0.92 | 0.70-1.21 | 0.534 | |||
|
| ||||||
| L estradiole | 1.01 | 0.93-1.10 | 0.826 | |||
|
| ||||||
| L FSHe | 1.03 | 0.91-1.16 | 0.661 | |||
|
| ||||||
| L Inhibin, be | 0.91 | 0.82-1.01 | 0.067 | |||
All models are adjusted for time in 3 categories: 1) premenopause: from 11 or more years to, but not including, the FMP; 2) FMP up to 3 years post FMP; 3) late postmenopause: >=3 years post FMP.
Odds ratio for interaction with time.
P value for interaction with time.
Odds ratio for each 1-point increase in score.
Odds ratio for each 1 SD change in log hormone value.
The percentage of poor sleepers in each time segment of the menopause transition is shown for each baseline severity group in Figure 2. The greatest proportion of moderate/severe poor sleepers around menopause were the women who reported moderate/severe poor sleep at the premenopausal baseline, with a prevalence of 54% averaged over the 11 years before the FMP, 47% averaged over the years around the FMP (FMP to 3y) and 44% averaged over the later years postmenopause (3 to 11y postmenopause). Among women who reported no poor sleep at the premenopausal baseline, 25% had moderate/severe poor sleep averaged over the 11 years before the FMP, 26% averaged over the years around the FMP, and 25% averaged over the later years postmenopause. In this group, the prevalence of poor sleep was flat over the menopause transition, with no significant increase around the FMP. Among women with mild poor sleep premenopause, 32% had moderate/severe poor sleep averaged over the 11 years before the FMP, 43% averaged over the years around the FMP (FMP to 3y) and 35% averaged over the later years postmenopause (3 to 10y postmenopause). Only in this last subgroup was there a significant increase in poor sleep around the FMP, which accounted for the interaction between baseline severity of poor sleep and moderate/severe poor sleep relative to the FMP (p=0.038 as shown in Table 3).
Figure 2.
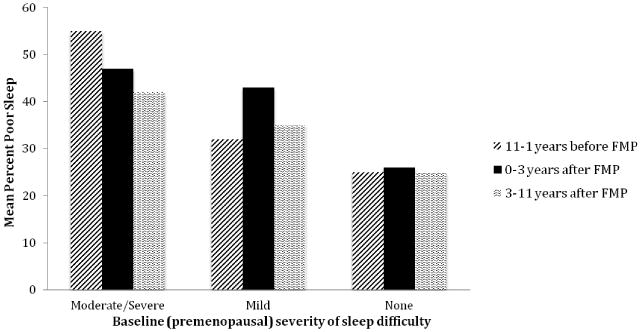
Percent of observations of poor sleep relative to the FMP by severity of poor sleep at the premenopausal baseline. The model included time defined in three segments: 1) from 11 or more years before but not including the FMP; 2) FMP up to 3 years post FMP; 3) >=3 years post FMP; baseline sleep severity (3 groups), moderate/severe hot flashes (yes, no) and the interaction of baseline sleep severity and hot flashes (P=0.038). Odds ratios are shown in Table 3.
Table 3. Unadjusted Associations Between Poor Sleep and Time Relative to Menopause Stratified by Sleep Severity at Baseline.
| Baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline Sleep Severity | 1-11y before FMP | FMP-3y post | >3y post FMP | |||||||
| N | OR | 95%CI | P | OR | 95%CI | P | OR | 95%CI | P | |
| None | 143 | 0.90 | 0.66-1.22 | 0.49 | 1.00 | 1.10 | 0.82-1.47 | 0.54 | ||
| Mild | 41 | 0.61 | 0.39-0.93 | 0.02 | 1.00 | 0.55 | 0.33-0.92 | 0.02 | ||
| Moderate/Severe | 71 | 1.09 | 0.79-1.50 | 0.62 | 1.00 | 0.75 | 0.50-1.11 | 0.15 | ||
Interaction of baseline severity × time, P=0.038.
The analyses were repeated to determine whether a later cutoff point for postmenopausal time relative to the FMP, i.e., 6 years postmenopause, altered the findings. The results were similar to those shown.
Hot flashes and poor sleep
The association of moderate/severe hot flashes with poor sleep relative to the FMP was highly significant in adjusted analysis (OR 1.79, 95% CI: 1.14-2.21, P<0.0001) (Table 2). This is illustrated in Figure 3, which shows that hot flashes increased around the FMP in each subgroup defined by sleep status at baseline. There was no significant interaction between hot flashes and baseline sleep status (P=0.25), indicating that hot flashes contributed to poor sleep similarly in each subgroup. There was also no significant interaction between hot flashes and time relative to menopause (P=0.69), indicating that hot flashes contributed to poor sleep similarly in each time period before, around and following the FMP. (interaction P=0.69). Figure 3 also shows that poor sleep occurred in the absence of hot flashes in all subgoups.
Figure 3.
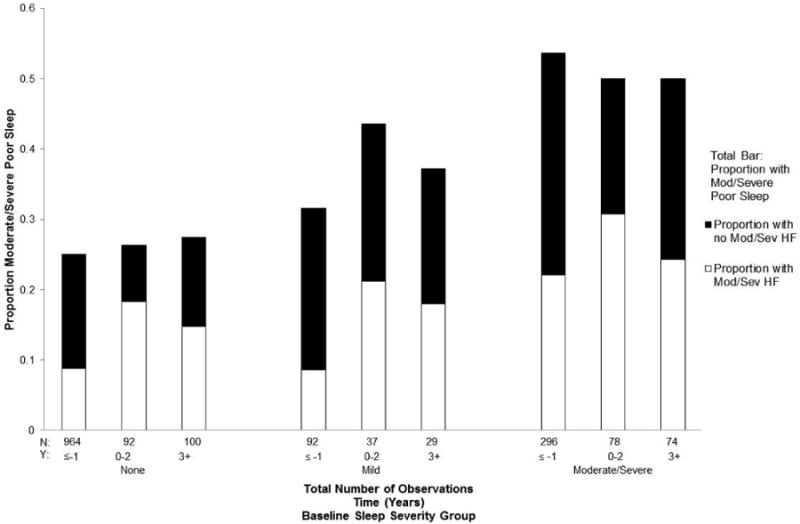
The non-significant interaction between severity of poor sleep at baseline (total bars) and moderate/severe hot flashes (white bars). The dark bars depict mild/no hot flashes. The model included baseline sleep severity (P<0.0001), hot flashes (P<0.0001), time (P=0.95) and the interaction of baseline sleep severity × hot flashes (P=0.25).
Other significant risk factors for moderate/severe poor sleep over the menopause transition included anxiety (P<0.0001) and perceived stress (P=0.016) in adjusted analysis (Table 2). The significant interaction between perceived stress and time indicated that the greatest impact of stress on poor sleep occurred in the time period prior to the FMP.
The hypothesized covariates of race, education, BMI, employment, alcohol use, smoking and age at FMP were not associated with moderate/severe poor sleep in either adjusted or unadjusted analysis. Hormone associations with poor sleep were not significant. Possibly this was due to the inclusion of time in these models, which reflected hormone changes in the same time interval.
Discussion
In this sample of mid-life women, the annual prevalence of moderate/severe poor sleep remained around 30% throughout the menopause transition, notably similar to the 30% prevalence of insomnia symptoms among adults in other population-based studies.1, 2 This yearly prevalence of poor sleep was relatively flat throughout the transition years, with no significant differences in any year relative to the FMP. The premenopausal sleep status was the strongest predictor of moderate/severe poor sleep in the menopause transition. Moderate/severe poor sleepers before the menopause transition continued to report moderate/severe poor sleep throughout the menopause transition, while women who did not have poor sleep before the menopause transition had the lowest risk of poor sleep in the menopause transition. These novel findings indicated that only one subgroup, which reported mild sleep problems at the premenopausal baseline, had a significant increase in poor sleep in the years around the FMP, suggesting a menopause-related form of sleep disturbance for a small “at risk” group of mid-life women.
Hot flashes contributed strongly to poor sleep regardless of sleep status before the menopause transition. This association was previously identified in studies of subjectively reported hot flashes, 4, 6, 10, 21 although was not found when both factors were measured physiologically,22 and the association remains controversial. In a large cross-sectional study where severe hot flashes were associated with chronic insomnia, approximately half of the women indicated that the insomnia preceded menopause.23 The researchers concluded that menopausal hot flashes influenced insomnia but that other factors such as chronic pain and poor health were important contributors regardless of menopausal status. Other studies that reported a high prevalence of poor sleep quality in mid-life women also found that symptoms occurring in the premenopause were strongly associated with poor sleep,24 but influences of age and menopause were modest.24, 25
Anxiety and perceived stress were significantly associated with poor sleep, although the causal direction of these associations remains an open question. Anxiety was identified as the strongest predictor of poor sleep quality in previous studies.26, 27 Depression was a risk factor for poor sleep but was not significant in adjusted analysis. Most participants were not clinically depressed and the measure of depression was highly correlated with anxiety, which had the stronger association with poor sleep in this study.
Reproductive hormone levels were not associated with poor sleep, as was found in some studies but not others,28, 29 suggesting that hormone levels were not strongly associated with poor sleep in healthy mid-life women. However, the lack of association in this study could also be due to our models, which included time relative to the FMP that reflected the changing hormone levels in the menopause transition. Reports of associations between poor sleep and hormone levels were significant when hormones were modeled without the inclusion of time periods.5, 10, 11 In our earlier reports on associations between menopausal status, hormone levels and sleep quality, we observed at baseline assessment that lower estradiol levels in women between the ages or 45-49 were associated with poorer sleep quality10 and that in 8-year follow-up, lower inhibin b levels, which were a marker of menopausal status, were an independent predictor of sleep difficulty.11 In another report that did not model time periods, there was an association of early follicular phase levels of estradiol and a more rapid rate of change in FSH with less favorable sleep quality in the early menopause transition.30
Limitations to consider include the subjective reports of poor sleep, which rated general sleep quality as perceived by the participants. The validity of the sleep item was previously demonstrated and had a high correlation with a standard measure of sleep quality.10, 11 Validity was further supported by the findings that showed the prevalence of moderate/severe poor sleep reported by the participants was nearly identical to the prevalence of symptomatic insomnia among adults as defined by the American Academy of Sleep Medicine.1 However, the sleep measure did not indicate diagnoses of sleep disorders such as sleep-disordered breathing and did not differentiate between different patterns of sleep difficulties, e.g, difficulty falling asleep versus mid-sleep awakenings versus early morning awakenings. We were unable to evaluate possible conditions underlying the sleep disturbances or whether the types of complaints associated with poor sleep changed over the reporting period. Also, the data were not sufficient to control for use of medications related to sleep in these models.
It is possible that including women who had sleep difficulties at the premenopausal baseline obscured information about poor sleep that first occurred in the menopause transition. Further studies that evaluate sleep disturbances that first occur in the menopause transition may identify whether there is a menopause-specific form of sleep disturbance. It is also possible that estradiol assays with a lower detectable limit than is measured by standard commercial assays would provide different results. However, the preponderance of observations in this study occurred before the FMP when estradiol levels were well above detectible limits, with only a small proportion of the observations in the late postmenopause, suggesting that more precise measures of the lowest estradiol levels would not change these non-significant results. This study also could not address the impact of hormone therapy or surgical menopause on sleep quality. Another study found that women using hormone therapy (HT) did not have significantly better sleep compared to those who did not use HT,31 and further studies to address these conditions are important for clinical care. Residual confounding due to potential predictors of sleep quality such as physical activity and other health and behavioral factors that may have a significant impact on sleep quality is another limitation of this study. These findings are based on a population-based cohort of African American and white urban women who were in general good health and may not be generalizable to all mid-life women, particularly those with major health problems that were not represented in this cohort.
The strength of this report is the longitudinal evaluation of poor sleep, commencing premenopause and extending into the postmenopausal years for all participants. This provided identification of the FMP with minimal recall bias and permitted analysis of sleep quality relative to the FMP in order to determine whether the pattern of poor sleep was associated with this marker of menopause. The population-based cohort was randomly identified and stratified to have similar numbers of African American and white women for analysis of racial associations. The measures of poor sleep, hormones, and covariates of the study were repeated annually to provide concurrent assessments at the premenopausal baseline and throughout the follow-up years.
Conclusions
Clinicians can expect that many mid-life women report sleep difficulties. However, clinicians should consider that poor sleep is not simply due to menopause or hot flashes, given the evidence that premenopausal sleep status was the strongest predictor of poor sleep in the menopause transition. Hot flashes were strongly associated with poor sleep as expected, but a large proportion of poor sleep in the menopause transition occurred without hot flashes. The common perception that poor sleep is associated with ovarian decline around menopause may pertain to only a small “at risk” subgroup, who had mild complaints at the premenopausal baseline that worsened around the FMP. Clinicians should obtain information on whether the onset of poor sleep occurred before the menopause transition as well as the severity and duration in order to formulate the most effective treatment strategies. Further studies that evaluate causes and treatments are needed to unravel this multifactorial problem and improve sleep health in mid-life women.
Acknowledgments
Supported by grants from the National Institutes of Health: RO1-AG-12745 (Dr. Freeman, Principal Investigator) and RR024134 (Clinical and Translational Research Center).
Dr. Freeman received research support from the National Institutes of Health, Forest Laboratories, Inc. and Bionovo. Dr. Sammel received grant support from the National Institutes of Health, consulted with and provided expert testimony for Swiss Precision Diagnostics.
Footnotes
Conflict of interest/disclosures: No other disclosures were reported.
References
- 1.The American Academy of Sleep Medicine. Insomnia. 2008 Available at: http://www.aasmnet.org. Retrieved April 14, 2014.
- 2.Roth T. Insomnia: definition, prevalence, etiology and consequences. J Clin Sleep Med. 2007;3(5Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson EO, Roth T, Schultz L, Breslau N. Epidemiology of DSM-IV insomnia in adolescence: lifetime prevalence, chronicity, and an emergent gender difference. Pediatrics. 2006;117:e247–256. doi: 10.1542/peds.2004-2629. [DOI] [PubMed] [Google Scholar]
- 4.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Kravitz HM, Zhao X, Bromberger JT, Gold EB, Hall MH, Matthews KA, et al. Sleep disturbance during the menopausal transition. Sleep. 2008;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 6.Ensrud KE, Stone KL, Blackwell TL, Sawaya GF, Tagliaferri M, Diem SJ, et al. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16:286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 7.Joffe H, White DP, Crawford SL, McCurnin KE, Edonomou N, Connors S, et al. Adverse effects of induced hot flashes on objectively recorded and subjectively reported sleep: results of a gonadotropin-releasing hormone agonist experimental protocol. Menopause. 2013;20:905–914. doi: 10.1097/GME.0b013e31828292d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young T, Rabago D, Zgierska A, Austin D, Finn L. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin sleep study. Sleep. 2003;26:667–672. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 9.Sharkey KM, Bearpark HM, Acebo C, Millman RP, Cavallo A, Carskadon MA. Effects of menopausal status on sleep in midlife women. Behav Sleep Med. 2003;1:69–80. doi: 10.1207/S15402010BSM0102_1. [DOI] [PubMed] [Google Scholar]
- 10.Hollander LE, Freeman EW, Sammel MD, Berlin JA, Grisso JA, Battistini M. Sleep quality, estradiol levels, and behavioral factors in late reproductive age women. Obstet Gynecol. 2001;98:391–397. doi: 10.1016/s0029-7844(01)01485-5. [DOI] [PubMed] [Google Scholar]
- 11.Pien GW, Sammel MD, Freeman EW, Lin H, DeBlassis TL. Predictors of sleep quality in women in the menopausal transition. Sleep. 2008;31:991–999. [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Liu L, Martin P. Psychometric properties of a menopausal symptom list. Menopause. 2003;10:258–265. doi: 10.1097/00042192-200310030-00014. [DOI] [PubMed] [Google Scholar]
- 13.Pilcher JJ, Ginter DR, Sadowsky B. Sleep quality versus sleep quantity: Relationships between sleep and measures of health, well-being and sleepiness in college students. J Psychosomatic Res. 1997;42:583–596. doi: 10.1016/s0022-3999(97)00004-4. [DOI] [PubMed] [Google Scholar]
- 14.Spitzer RL, Williams JB, Kroenke K, Linzer M, deGruy FV, 3rd, Hahn SR, et al. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA. 1994;272:1749–1756. [PubMed] [Google Scholar]
- 15.Zung WWK. A rating instrument for anxiety disorders. Psychometrics. 1971;12:371–379. doi: 10.1016/S0033-3182(71)71479-0. [DOI] [PubMed] [Google Scholar]
- 16.Radloff LS. The CES-D scale: a self-report despression scale for research in the general population. Applied Psychological Measurements. 1977;1:385–401. [Google Scholar]
- 17.Cohen S, Kamarck TW, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 18.Liang KY, Leger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 19.Freeman EW, Sammel MD, Sanders RJ. Risk of long-term hot flashes after natural menopause: evidence from the Penn ovarian aging study. Menopause. 2014;21 doi: 10.1097/GME.0000000000000196. Jan 27 (E pub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar SW, et al. Executive summary of the Stages of Reproductive Aging Workship + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metabl. 2012:1159–1168. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu H, Thurston RC, Matthews KA, Bryce CL, Hays RD, Kapoor WN, et al. Are hot flashes associated with sleep disturbance during midlife? Results from the STRIDE cohort study. Maturitas. 2012;71:34–38. doi: 10.1016/j.maturitas.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurston RC, Santoro N, Matthews KA. Are vasomotor symptoms associated with sleep characteristics among symptomatic midlife women? Comparisons of self-report and objective measures. Menopause. 2012;19:742–748. doi: 10.1097/gme.0b013e3182422973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oyayon MM. Severe hot flashes are associated with chronic insomnia. Arch Int Med. 2006;166:1262–1268. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 24.Blumel JE, Cano A, Mezones-Holguin E, Baron G, Bencosme A, Benitiz Z, et al. A multinational study of sleep disorders during female mid-life. Maturitas. 2012;72:359–366. doi: 10.1016/j.maturitas.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Tom SE, Kuh D, Guralnik JM, Mishra GD. Self-reported sleep difficulty during the menopausal transition: results from a prospective cohort study. Menopause. 2010;17:1128–1135. doi: 10.1097/gme.0b013e3181dd55b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:1–4. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 27.Cheng MH, Hsu CY, Wang SJ, Lee SJ, Wang PH, Fuh JL. The relationship of self-reported sleep disturbance, mood and menopause in a community study. Menopause. 2008;15:958–962. doi: 10.1097/gme.0b013e318160dafa. [DOI] [PubMed] [Google Scholar]
- 28.Moreno-Frias C, Figueroa-Vega N, Malacara JM. Relationship of sleep alterations with perimenopausal and postmenopausal symptoms. Menopause. 2014;21 doi: 10.1097/GME.0000000000000206. Feb 24 (E pub ahead of print) [DOI] [PubMed] [Google Scholar]
- 29.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle midlife women's health study. Sleep. 2010;33:539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sowers MF, Zheng H, Kravitz HM, Matthews K, Bromberger Jt, Gold EB, et al. Sex steroid hormone profiles are related to sleep measures from polysomnography and the Pittsburgh sleep quality index. Sleep. 2008;31:1339–1349. [PMC free article] [PubMed] [Google Scholar]
- 31.Xu M, Belanger L, Ivers H, Guay B, Zhang J, Morin CM. Comparison of subjective and objective sleep quality in menopausal and non-menopausal women with insomnia. Sleep Medicine. 2011;12:65–69. doi: 10.1016/j.sleep.2010.09.003. [DOI] [PubMed] [Google Scholar]


