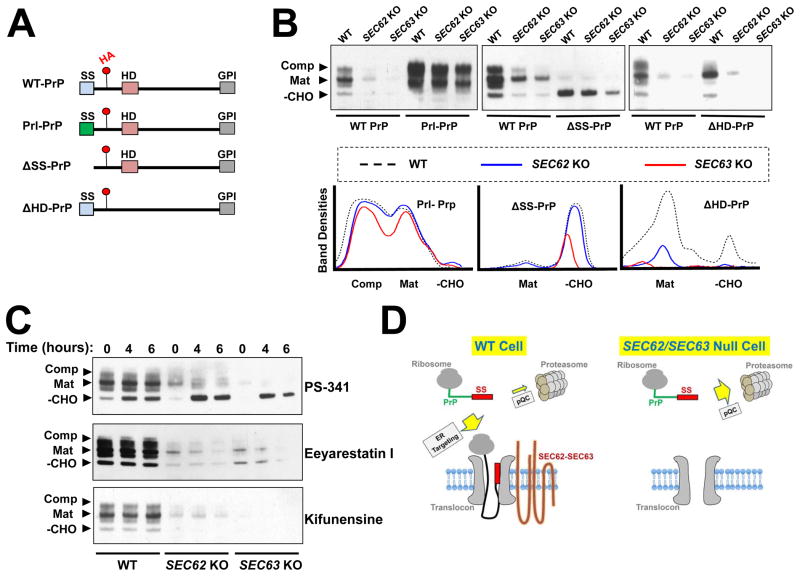
Figure 6. The signal sequence of PrP dictates the choice between ER targeting and cytosolic degradation.
(A) Diagrams showing WT and mutant PrP proteins used in this study with key domains indicated. Red dots indicate the position of an HA tag in an unstructured domain of PrP. SS: signal sequence; HD: hydrophobic domain; GPI: C-terminal GPI-conjugating sequence. (B) Immunoblots showing the expression of WT and mutant PrP proteins. Plasmids encoding the indicated proteins were transiently expressed in WT and mutant HAP1 cells and were detected by immunoblotting using monoclonal anti-HA antibodies. Mutant HAP1 cells deficient in SEC62 or SEC63 were prepared as described in Figure 5. –CHO: the cytosolic/immature form; Mat: the fully glycosylated mature form; Comp: the fully glycosylated complexed form (Kretzschmar et al., 1986, Emerman et al., 2010a). (C) WT PrP was transiently expressed in WT or mutant HAP1 cells. The cells were treated with 100 nM of the proteasome inhibitor PS-341, 10 μM of the ERAD inhibitor Eeyarestatin I, or 1 μM of the ERAD inhibitor kifunensine for the indicated periods of time before analysis by immunoblotting. (D) Model illustrating the dual role of signal sequence in the PrP pathway. In WT cells, the signal sequence is recognized by SEC62-SEC63 and targeted to the ER. In the absence of SEC62-SEC63, the signal sequence directs PrP to the pQC pathway for degradation.