Figure 2. The apoptotic CED pathway is required for elimination of presynaptic components.
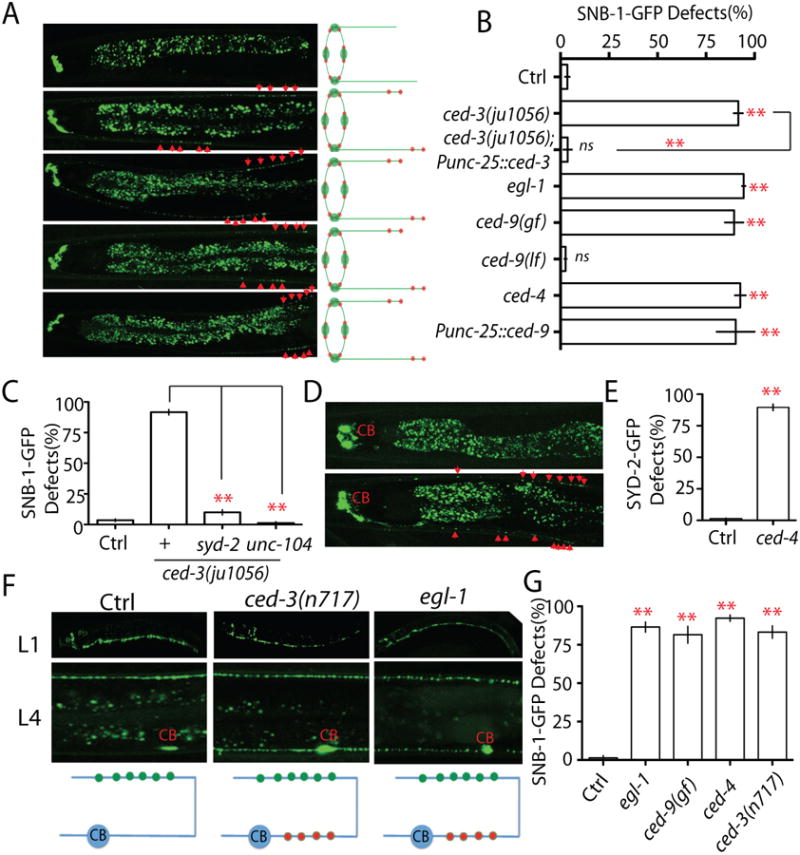
(A-B) Loss-of-function in the CED pathway prevents elimination of presynaptic components. (A) Images and schematics of the localization of synaptic vesicle puncta in wild type and mutant animals. (B) Quantification of SNB-1-GFP elimination defects (% animals) in different genotypes. Punc-25 promoter was used to express genes in RME neurons; unc-30(ju32) animals were used as background to eliminate unc-25 expression in the D neurons. The strain information is listed in Table S1 and Table S2. (C) Quantification data show that loss-of-function in syd-2 and unc-104 suppress SNB-1-GFP elimination defects in ced-3(lf) animals. (D-E) loss-of-function in ced-4 suppressed elimination of presynaptic active zones. (D) Images and schematics of the localization of active zone puncta (Punc-25∷SYD-2-GFP). (E) Quantification of active zone elimination defects. (F-G) The CED Pathway regulates synapse elimination in DD motor neutrons. (F) Images and schematics of the localization of synaptic puncta (Pflp-13-SNB-1∷GFP/juIs137) in wild type, ced-3(lf) and egl-1(lf) animals at L1 and L4 stages. CB, cell bodies; green dot, new synapses at the dorsal cord; red dot, synapses at the ventral cord. (G) Graph shows the quantification data of DD motor neuron synapse elimination defects in L4 animals. In Figure B, E and G, experiments were performed at least 3 times, with N ≥80 animals each time. For transgenic animals the results shown here are generated from at least three independent lines. Data is shown as mean ± SD. Student test, ** P < .01, ns: no significant difference. Scale bar, 10 μm. See also Supplemental Figure 1.
