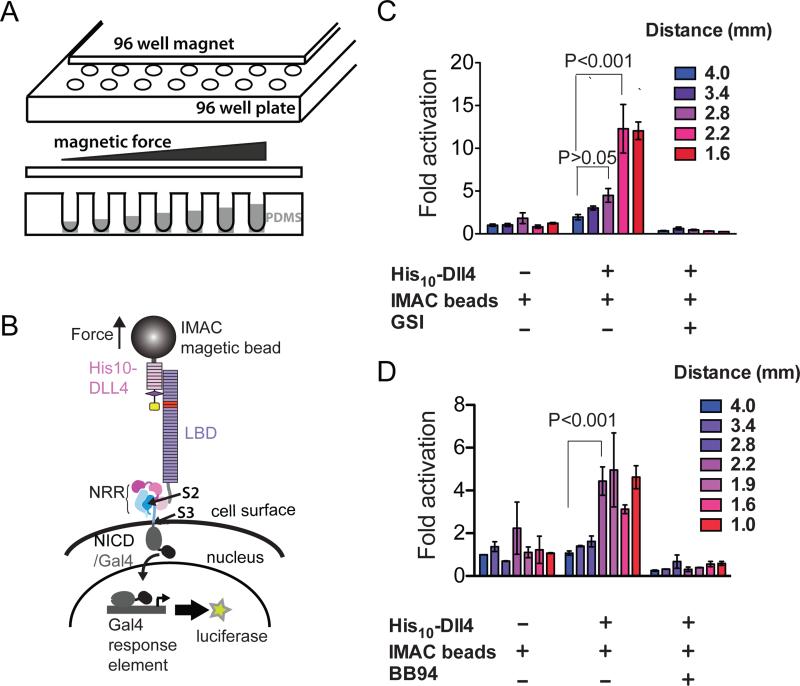
Figure 2. Response of cell-surface Notch receptors to applied force using a multiplexed magnetic tweezers assay.
(A) Experimental design. A plate containing 96 cylindrical magnets is positioned over a 96 well plate of cells in order to apply force to magnetic beads tethered to Notch molecules on the cell-surface. The distance between the cells and the magnet is varied by using the polymer PDMS to create terraces of different heights. (B) Assay schematic. Cells expressing Notch1 receptors in which the ankyrin-repeat domain has been replaced by the Gal4 DNA-binding domain (Malecki et al., 2006) are stimulated by magnetic beads loaded with the ligand DLL4, followed by measurement of luciferase reporter gene activity. (C, D) Luciferase reporter gene activity in response to various treatments as a function of the distance from the magnet. U2OS cells expressing Notch1-Gal4 receptors were incubated with magnetic beads alone or beads loaded with the ligand DLL4 in the absence or presence of a gamma secretase inhibitor (GSI) (C) or metalloprotease inhibitor BB94 (D). Luciferase reporter gene activity is reported relative to the response of cells to beads alone at a distance of 4 mm from the magnet. Error bars represent the standard error of the mean of triplicate measurements, and statistical significance was determined with a two-way ANOVA followed by a post-hoc Bonferroni's test. 96-well magnet calibration is provided in Figure S2.