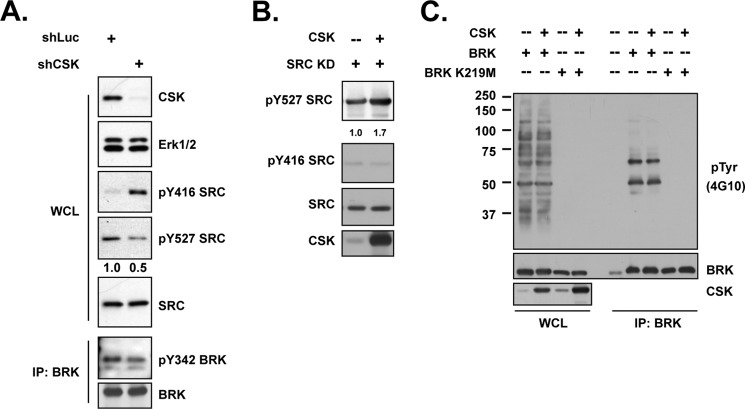
FIGURE 4.
Activity of SRC, but not BRK, was negatively regulated by CSK. (A) (upper) T47D breast cancer cells, expressing either luciferase control or CSK shRNA, were lysed and immunoblotted with the indicated antibodies to determine the activation status of SRC. Knockdown efficiency was illustrated by blotting with anti-CSK antibody, and ERK1/2 was probed as loading control. (Lower) Immunoprecipitation followed by blotting analysis were applied to same lysates to check the activation of BRK upon CSK knockdown. (B) 293T cells were transiently transfected with SRC kinase-dead mutant and CSK expression plasmids, as indicated, and phosphorylation changes on SRC Tyr-527 and Tyr-416 were determined by phosphospecific antibodies. The intensity of the signal in the SRC Tyr(P)-527 blot was normalized to total SRC and quantitated by ImageJ. (C) Indicated constructs were transiently transfected in 293T cells. BRK was immunoprecipitated, and its activation status was examined by probing with specific Tyr-342 and Tyr(P) (4G10) antibodies.