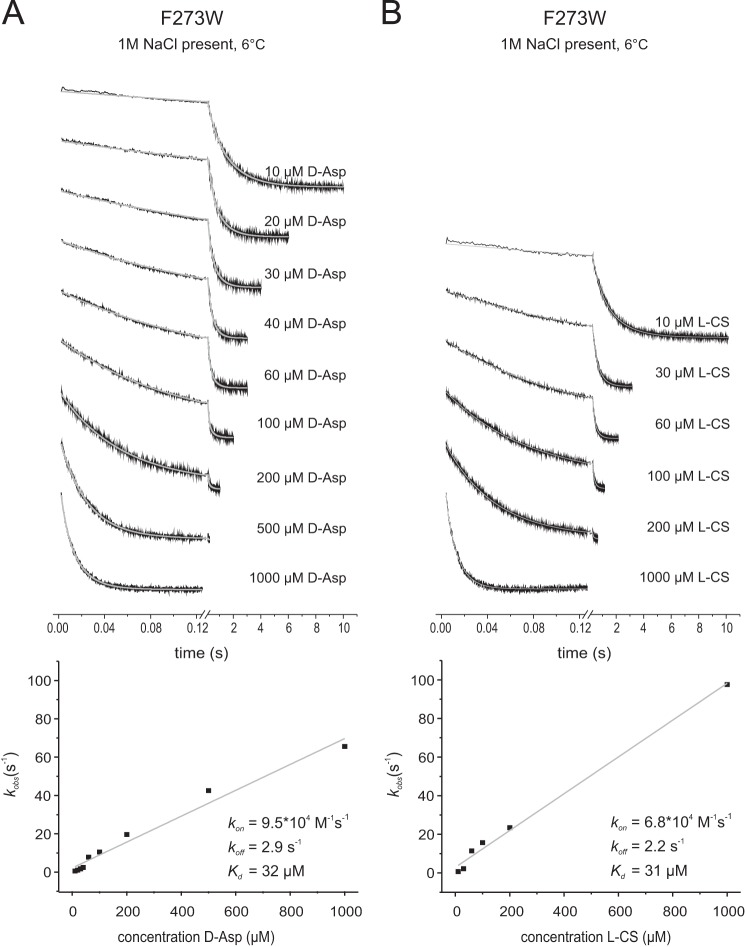
FIGURE 9.
Rates of d-aspartate and l-cysteine sulfinic acid binding to GltPh F273W in the presence of 1 m NaCl. The top panels show the stopped-flow fluorescence measurements. Each trace (black) represents an average of 3–8 individual traces, fitted with a double exponential equation (gray). In the bottom panels the kobs values resulting from the fits with assumed pseudo-first-order reaction are plotted against the corresponding substrate concentrations. All measurements were performed at 6 °C. The slopes of the linear fits give the kon, and the intercepts with the kobs axis give the koff values. A, 1.5 μm F273W was mixed with varying d-Asp concentrations as indicated. B, 1.5 μm F273W was mixed with varying l-cysteine sulfinic acid (l-CS) concentrations as indicated.