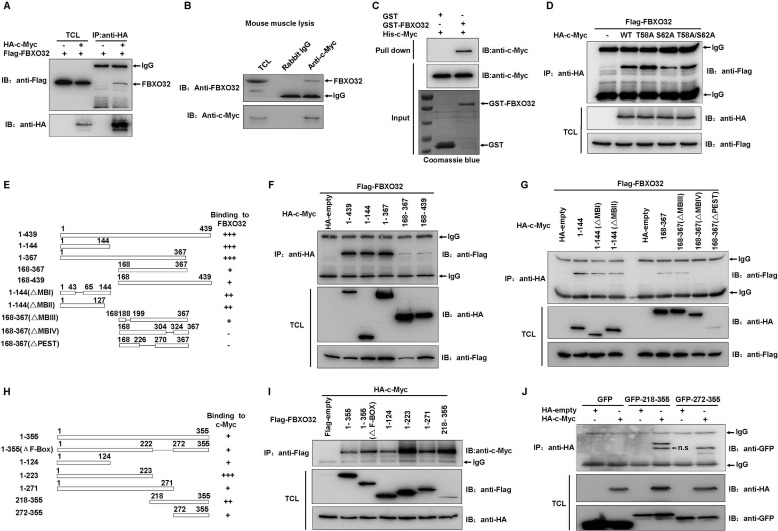
FIGURE 3.
FBXO32 directly interacts with c-Myc. A, co-immunoprecipitation assays show FLAG-tagged FBXO32 interacts with HA-tagged c-Myc when they are overexpressed in HEK293T cells. B, endogenous FBXO32 interacts with endogenous c-Myc, as revealed by co-immunoprecipitation assays using anti-c-Myc antibody in mouse muscle lysates. Rabbit IgG is used as control for co-immunoprecipitation assays. C, bacterial expressed GST-tagged FBXO32 directly interacts with bacterial expressed His-tagged c-Myc. Bacterial expressed GST protein is used as control. D, the wild-type c-Myc as well as its mutants T58A, S62A, and T58A/S62A interact with FBXO32, as revealed by co-immunoprecipitation assays. E, schematic of the c-Myc domains. The extent of the interaction between FBXO32 and the c-Myc domains is indicated by the number of plus signs. F and G, co-immunoprecipitation of human FLAG-FBXO32 with HA-tagged c-Myc domains in HEK293T cells transfected with the indicated plasmids. H, schematic of the FBXO32 domains. The extent of the interaction between c-Myc and the FBXO32 domains is indicated by the number of plus signs. I, co-immunoprecipitation of human HA-c-Myc with FLAG-tagged FBXO32 domains in HEK293T cells transfected with the indicated plasmids. J, co-immunoprecipitation of human HA-c-Myc with GFP-tagged FBXO32 domains in HEK293T cells transfected with the indicated plasmids. IP, immunoprecipitation; IB, immunoblot; n.s., nonspecific; TCL, total cell lysate.