FIGURE 2.
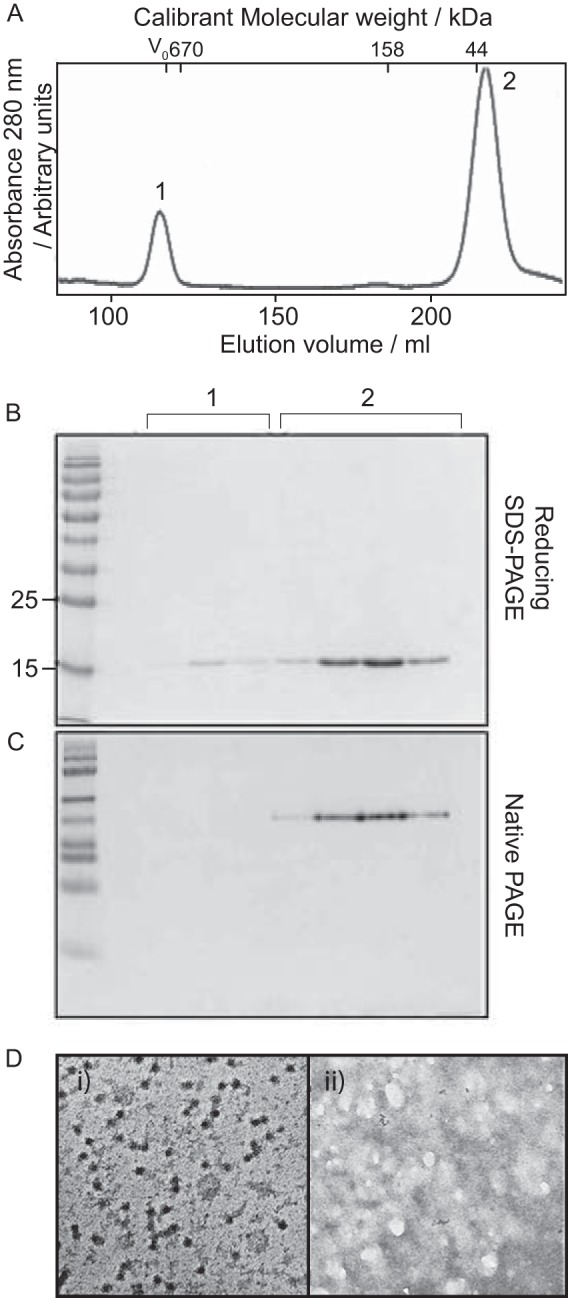
Disassembly of capsids composed of WT Cp. Protein at ∼2 mg/ml was disassembled with 3 m urea at pH 9.6. A, chromatogram of protein separation on a Superdex 200 column (capsid and Cp peaks are indicated as Peaks 1 and 2, respectively). B, reducing SDS-PAGE of peak fractions. Both peaks contained protein of the same molecular weight, corresponding to the expected size of WT Cp (17.3 kDa) by comparison to the standards shown (LHS). C, native PAGE of peak fractions. Protein from the capsid peak did not enter the gel matrix, however, the Cp peak is well resolved to a single species. D, TEM (negative stain) of (i) capsid peak (ii) Cp peak. Higher-order structures are visible in (i) however, (ii) is devoid of these. Bar, 100 nm.
