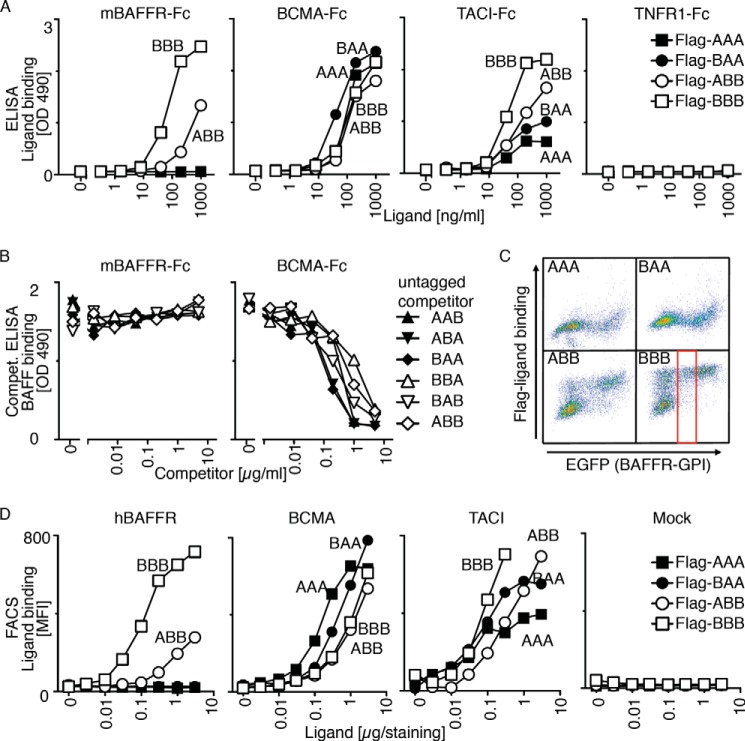
FIGURE 4.
Receptor binding specificity of BAFF-APRIL heteromers. A, binding of titrated amount of FLAG-tagged single-chain BAFF-APRIL heteromers to the indicated immobilized receptors-Fc was monitored in an ELISA-based assay. B, binding of a constant, nonsaturating amount of FLAG-BAFF to mBAFFR-Fc or BCMA-Fc was monitored in an ELISA-based assay. Before the addition of FLAG-BAFF, coated receptor Fcs were preincubated with titrated amounts of the indicated untagged single-chain BAFF-APRIL heteromers that served as competitors for FLAG-BAFF. C, 293T cells were co-transfected with plasmids coding for the following: (a) the extracellular domain of hBAFFR fused to the C-terminal GPI addition signal of TRAILR3, and (b) EGFP as a fluorescent tracer. These cells were stained with the indicated FLAG-tagged single-chain BAFF-APRIL heteromers (at 1 μg/staining = 40 μg/ml). FACS scattergrams show the binding of ligands (y axis) as a function of EGFP expression (which itself is proportional to BAFFR-GPI). Both axes show fluorescence intensities (from 100 to 104 arbitrary units) on a log scale. The gate shows the population of cells with intermediate EGFP expression that was selected to quantify mean fluorescence intensity in D. D, mean fluorescence intensity (MFI) of the binding of titrated amounts of the indicated single-chain FLAG-tagged heteromers to 293T cells expressing hBAFFR-GPI, BCMA-GPI, TACI-GPI or no transfected receptor (Mock). Experiment was performed as described in C. Experiments shown in A–D are representative of at least two with similar results.