FIGURE 3.
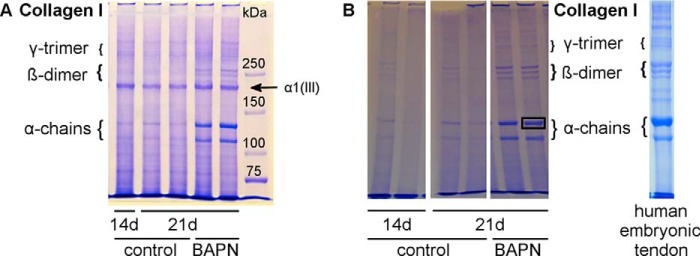
SDS-PAGE analysis of collagen chains. A, equal amounts of tendon constructs were analyzed by SDS-PAGE to assess the collagen cross-link abundance in 14- and 21-day controls versus BAPN treatment. The characteristic collagen type I pattern is shown with α-monomers, β-dimers, and γ-trimers. Overall, the BAPN samples show higher band intensity, particularly for monomers and dimers, suggesting higher collagen solubility due to fewer intermolecular covalent cross-links. Furthermore, the prominent collagen pN α1(III) chain is indicated. This is present in similar amounts per construct for all samples, even in the controls in which collagen type I chains are noticeably less soluble and abundant in the extracts due to lysyl oxidase cross-linking. B, SDS-PAGE without DTT sample reduction of tendon constructs and human embryonic tendon. Similar bands are seen as in A but without the pNα1(III) band. The human embryonic tendon extract shows a similar pattern to the tendon constructs. Single bands were excised for mass spectrometry analysis as indicated in the right 21d BAPN lane.
