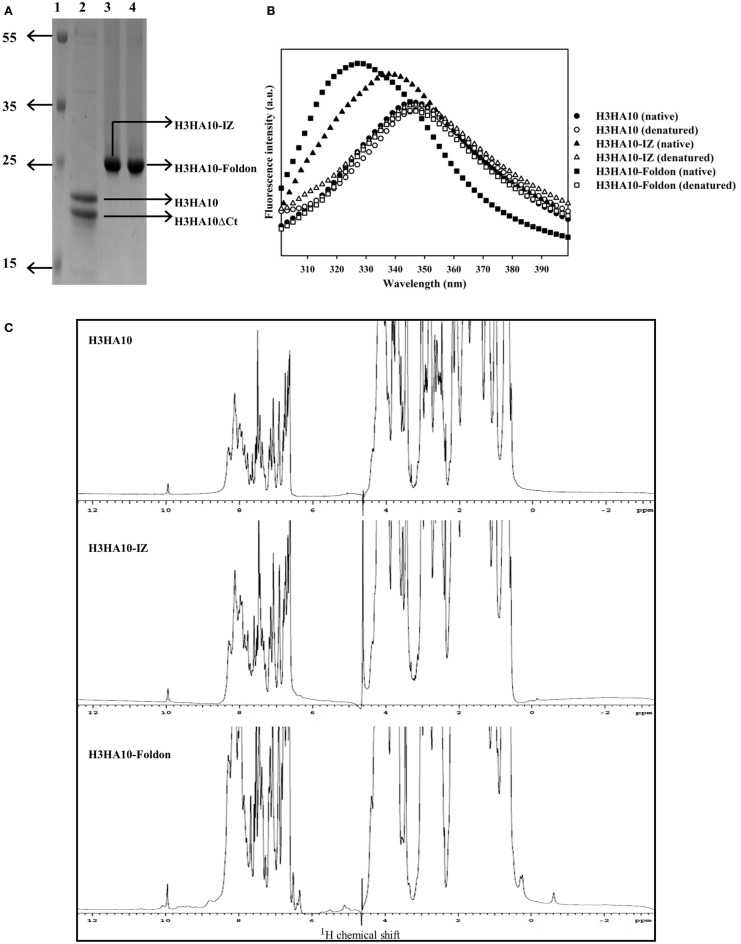
Figure 2.
Protein purification and biophysical characterization of HA stem-fragment immunogens. (A) SDS-PAGE profile of the purified proteins. Lane 1: PageRuler Plus prestained protein ladder (Thermo Scientific), lane 2: H3HA10, lane 3: H3HA10-IZ, and lane 4: H3HA10-Foldon. All the designed proteins were purified from the soluble fraction of E. coli cell culture lysate. H3HA10 was partially degraded upon purification. A C-terminal cleavage of 1367.4 Da (determined by mass spectrometry) was observed (H3HA10ΔCt = 16335.9 Da). The derivative(s) of H3HA10 with C-terminal trimerization motif(s) were resistant to in situ protein degradation. The migration of the purified proteins in a SDS-PAGE is marginally retarded. The SDS-PAGE gel was stained with Coomassie. (B) Fluorescence emission spectra of HA stem-fragment immunogens were recorded under native (PBS, pH 7.4) or denaturing conditions (7M GdmCl in PBS, pH 7.4) as indicated. Unlike H3HA10, both H3HA10-IZ and H3HA10-Foldon showed a significant red-shift in the emission maxima upon denaturation indicating a compact tertiary conformation. (C) 1D 1H NMR spectra of HA stem-fragment immunogens. The improved chemical shift dispersion in the upfield (0.5–1.0 ppm) and/or downfield (9–11 ppm) regions of the 1H NMR spectra of H3HA10-IZ and H3HA10-Foldon is consistent with the fluorescence data, indicating that trimerization motifs assist in the folding of the HA stem in the absence of the transmembrane (TM) domain, with H3HA10-Foldon appearing more structured than H3HA10-IZ.