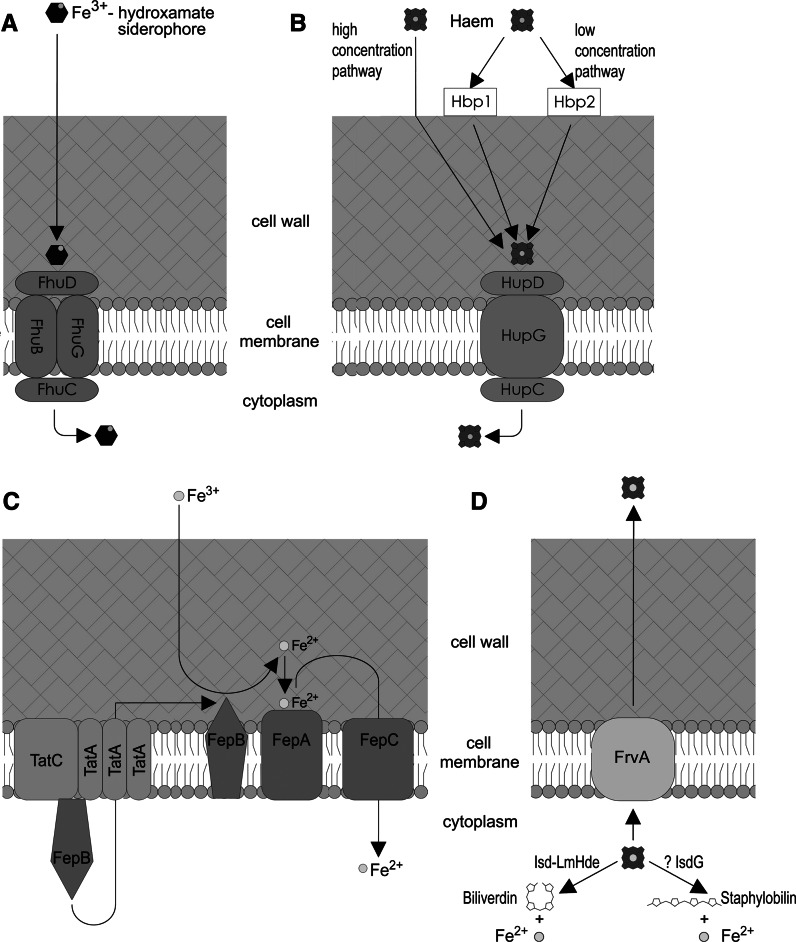
Fig. 1.
Systems of iron transport in L. monocytogenes a Transport of hydroxamate siderophores. The transport system consists of FhuD receptor protein, membrane permeases FhuB and FhuG and protein FhuC which is the ATP binding component of the system. b Transport of haem. Sortase-independent transport of haem involves the HupD receptor, membrane permease HupG and protein HupC, which is the ATP binding component of the system. Sortase-dependent transport of haem takes place under conditions of low extracellular concentrations of haem (<50 nM). In this case, in addition to proteins HupDCG, the process of haem acquisition involves proteins Hbp1 and Hbp2, which are responsible for capturing porphyrin from the environment. c Reductive iron uptake. In the proposed model FepB is translocated across the membrane by Tat translocon. At extracellular surface of membrane FepB acts as the ferric reductase enzyme. After reduction, ferrous ions are bound by the iron binding lipoprotein FepA, and then are transported into the cell by ferrous permease FepC. d Export of haem. Haem present in excess is exported to the external environment most probably with the involvement of protein FrvA. Catabolic pathway of exogenous haem in L. monocytogenes cells is also shown. Haem acquired from the external environment is degraded by Isd-LmHde enzyme to free iron and biliverdin or, would be degraded by IsgG protein to staphylobilin and Fe2+