Figure 1. Subject Randomization and Follow-Up.
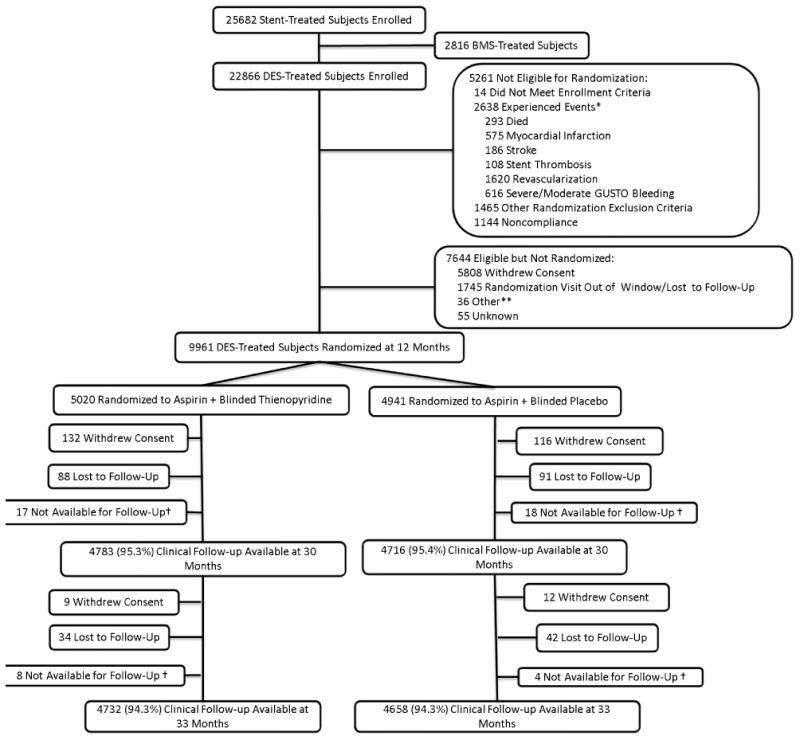
Subjects were enrolled within 72 hours of stent placement, followed for 12 months on open-label thienopyridine plus aspirin, then randomized to 18 months of thienopyridine or placebo (each in addition to aspirin). Randomized treatment ended at 30 months and subjects remained on aspirin only and were followed for another 3 months. While the number of subjects with clinical follow-up available is reported in each arm, the co-primary efficacy endpoints were analyzed according to the principle of intention to treat, including all randomized subjects and last available follow-up information.
*Subjects may have >1 event.
**Site terminated participation, randomization target met prior to subject follow-up, or subject not recognized to be eligible by site
† Subjects moved, were incarcerated, or were prematurely exited from the study.
