Abstract
Cardiovascular disease (CVD) constitutes one of the major causes of deaths and disabilities, globally claiming 17.3 million lives a year. Incidence of CVD is expected to rise to 25 million by 2030, and Saudi Arabia, already witnessing a rapid rise in CVDs, is no exception. Statins are the drugs of choice in established CVDs. In the recent past, evidence was increasingly suggesting benefits in primary prevention. But over the last decade Saudi Arabia has a witnessed significant rise in CVD-related deaths. Smoking, high-fat, low-fiber dietary intake, lack of exercise, sedentary life, high blood cholesterol and glucose levels were reported as frequent CVD-risk factors among Saudis, who may therefore be considered for primary prevention with statin. The prevalence of dyslipidemia, in particular, indicates that treatment should be directed at reducing the disorder with lipid-modifying agents and therapeutic lifestyle changes.
The recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines has reported lowering the low-density lipoprotein cholesterol (LDL-C) target levels, prescribed by the 2011 European Society of Cardiology (ESC)/the European Atherosclerosis Society (EAS). The new ACC/AHA guidelines have overemphasized the use of statin while ignoring lipid targets, and have recommended primary prevention with moderate-intensity statin to individuals with diabetes aged 40–75 years and with LDL-C 70–189 mg/dL. Treatment with statin was based on estimated 10-year atherosclerotic-CVD (ASCVD) risk in individuals aged 40–75 years with LDL-C 70 to 189 mg/dL and without clinical ASCVD or diabetes. Adoption of the recent ACC/AHA guidelines will lead to inclusion of a large population for primary prevention with statins, and would cause over treatment to some who actually would not need statin therapy but instead should have been recommended lifestyle modifications. Furthermore, adoption of this guideline may potentially increase the incidences of statin intolerance and side-effects. On the other hand, the most widely used lipid management guideline, the 2011 ESC/EAC guidelines, targets lipid levels at different stages of disease activity before recommending statins. Hence, the 2011 ESC/EAC still offers a holistic and pragmatic approach to treating lipid abnormalities in CVD. Therefore, it is the 2011 ESC/EAC guidelines, and not the recent ACC/AHA guidelines, that should be adopted to draw guidance on primary prevention of CVD in Saudi Arabia.
Keywords: Primary prevention, Statins, Cardiovascular disease, Atherosclerotic-cardiovascular disease, ACC/AHA guidelines
Abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- ASCVD
atherosclerotic-CVD
- CHD
congestive heart disease
- CRP
C-reactive proteins
- CVD
cardiovascular disease
- EAS
European Atherosclerosis Society
- ESC
European Society on Cardiology
- HDL
high density lipoprotein
- HMG-CoA
3-hydroxyl-3-methylglutaryl coenzyme A
- LDL
low density lipoprotein
- MI
myocardial infarction
- NCEP-ATP
National Cholesterol Education Program Adult Treatment Panel
- RCT
randomised clinical trials
- SCORE
Systematic Coronary Risk Estimation
Introduction
Cardiovascular disease (CVD) is one of the most prevalent diseases in healthy men and women and globally a major cause of death and disability. According to a report, CVD including heart attacks and strokes claim the lives of 17.3 million people every year. It has been estimated that this figure is likely to reach 25 million people in 2030 if no effective interventions are available [1]. A study from the Middle East reports that the rate of increase in CVD-associated mortalities is one of the highest in the world [2]. In Saudi Arabia, CVD accounts for over 22% of deaths each year, and other estimates showed over 42% of all deaths are attributed to CVD in Saudi Arabia [3,4]. Another study from Saudi Arabia reports a similar prevalence pattern of diseases to the West, with cardiac and respiratory disorders reported as the most frequent, and diabetes and ischemic heart disease as the main causes for hospitalization [5]. In oil-rich Middle Eastern countries there have been drastic changes in lifestyles accompanied by several CVD-risk factors which include smoking, eating high fat (especially red meat) and low-fiber diets, increased waist-to-hip ratio, and increased blood cholesterol and sugar, among other factors. Most of the young generation of Saudis have multiple risk factors. Statins provide proven benefits in the secondary prevention of CVDs while accumulating reports suggest statins also provide primary prevention of CVD [6]. The present review discusses statins in the primary prevention of CVD in Saudi Arabia in light of current and past lipid management guidelines.
Statins and their mechanism of action
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are a class of lipid lowering drugs that have revolutionized CVD pharmacotherapy. This class of lipid lowering agents competitively inhibit the rate limiting step in lipid biosynthesis, i.e., conversion of HMG-CoA to mevalonate, leading to the prevention of cholesterol biosynthesis. Over the past few years, the medical literature has reported that statins could reduce vascular inflammation and the development of atherosclerosis through a mechanism independent of their lipid lowering effects, referred to as “pleiotropic effects” (Fig. 1).
Figure 1.
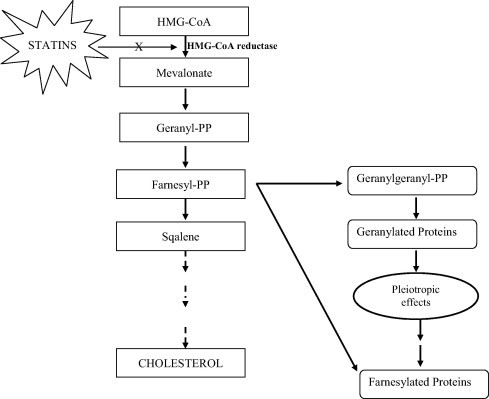
Diagrammatic elucidation of mechanisms of statins; HMG-CoA: hydroxyl methyl glutaryl-coenzyme A; PP: pyrophopspahte.
The lipid-independent effects of statins are reported to result from inhibition of isoprenoids (geranylgeranyl pyrophosphate), the products of which are important lipid attachments for a host of intracellular signaling proteins [7] and hence inhibit the activation of various signaling proteins which include heme A, nuclear lamins, and small guanosine triphosphate bound Ras and Ras-like proteins, which include Rho, Rac and Cdc42, and the γ-subunit of heterotrimeric G-proteins [8]. These proteins are reported as critical for controlling a host of cellular functions, which include cellular growth and differentiation, expression of genes, cytoskeletal assembly, and cell motility in the transport of proteins and lipids, nuclear transport, and cellular immunity. Statins were reported to display anti-inflammatory, antioxidant, antiplatelet, antiproliferative, and immunosuppressive properties [9–11]. Statins cause plaque stability through combined reduction of lipids, macrophages, and matrix metalloproteinases, and prevent endothelial dysfunction by inhibiting isoprenylation of Rac and Rho, and activation of the Rho-associated kinases pathway [12,13].
Primary prevention with statins in cardiovascular diseases
The clinical and preclinical literature is replete with findings that treatment with statins significantly reduces occurrence of deaths and disabilities in patients with known clinical manifestation of CVD [14–16]. Statins, therefore, are widely used and are the most effective hypolipidemic agents available to clinicians for the reduction of cardiovascular risks and prevention of the worsening of disease/disability in patients with established CVD (secondary prevention). Primary prevention refers to interventions that aim to assess and manage cardiovascular risk in people who have not yet developed or clinically manifested CVD which may further help to prevent future disease in asymptomatic individuals with risk factors. In primary prevention strategy, statin use has remained a debatable topic in patients at relatively low risk and without the manifestation of CVD. However, a meta-analysis of a large statin trial reported over one-fourth relative reduction in the risk of cardiovascular events in both primary and secondary prevention with statin, and also in patients with varying risk factor profiles [15]. The guidelines on cholesterol treatment recommends use of statin therapy in primary prevention based on CVD profile and low density lipoprotein (LDL)-C level of patients [17]. In addition, the guidelines recommend statin therapy only in patients with average or below average risk for diabetes mellitus (with LDL-C levels <160 mg/dL), and two or more risk factors [14]. In 2009, Brugts et al. [16] reported a meta-analysis of 10 randomized clinical trials (RCT) that involved 16,078 and 23,681 men and women, respectively, with diabetes mellitus, and had a mean follow-up of 4.1 years. The findings of the meta-analysis showed that there was a considerable lowering of all cause mortality, major coronary and cerebrovascular events with the treatment of statin, and that the statin treatment did not increase the risk of cancer. No significant differences were observed with respect to the treatment effects in the study sub-populations. Hence, it was concluded that in asymptomatic patients with cardiovascular risk factors, treatment with statin considerably increased survival rate, and showed greater reduction in the risk of major cardiovascular events [16]. However, in day-to-day clinical practices, statin therapy is reported as costly for primary prevention in individuals considered to be at low risk of vascular disease, despite the fact that generic statins are available at considerably low costs. It was suggested that the cost of statin therapy in primary prevention could be reduced through strict adherence to statin therapy [18].
In 2011, Taylor et al. [19] reported a meta-analysis for primary prevention with statins. The study involved 14 randomized control trials conducted from 1994 to 2006. In this study, all randomized control trials compared statins with the dummy or usual care, and consisted of ⩽1 year treatment and a six-month follow-up period. The findings of the meta-analysis revealed a significant reduction in statin side effects, muscle pain, including cancer. The study also showed reduction in all-cause mortality and revascularization process with statin treatment in individuals without manifestations of CVD. Recently, Taylor et al. [20] reported another systematic review that included 18 RCTs with statins. The study compared statin treatment with a dummy or usual care control with ⩽1 year and a six-month follow-up period in adults without restrictions on total, LDL, or high density lipoprotein (HDL) cholesterol levels, and where 10% or less of study participants had a history of CVD. However, the study included only 14 clinical studies that involved study participants with particular disease conditions such as hypercholesterolemia, diabetes, hypertension, and microalbuminuria. It was observed that statins reduced all-cause mortality, relative risk of combined fatal and non-fatal congestive heart disease (CHD), stroke, and CVD. There was a reduction of revascularization rates. Total cholesterol and LDL-C were reported to have been reduced in all the studies. The authors did not find any deleterious effects with the use of statins. Further, they were of the opinion that statins for primary prevention could be worthwhile and would improve quality of life in patients [20]. The Cholesterol Treatment Trialists study that included individual patient data report indicated that the benefits accrued with statin use were similar in people at lower (less than one percent per year) risk of a major cardiovascular event. Therefore, the study supported findings that statins reduced all-cause mortality, major vascular events and revascularizations in individuals without clinical manifestation of CVD [20].
In 2012, de Vries et al. reported that primary prevention with statins among diabetic patients had a significant beneficial effect on event rates for first-time occurrence of a major cardiovascular or cerebrovascular event, fatal/non-fatal stroke and fatal/non-fatal myocardial infarction (MI) [21]. A review of the literature on the primary prevention of statins reported considerable reduction in cardiovascular morbidity over the short and long term, and a general trend towards a reduction in mortality over the long term. Taken together, these data provided some of the strongest evidence supporting statin use in primary prevention in individuals with cardiovascular risk factors for developing CHD over the next 10 years [22].
Two meta-analyses [24] published after 2012 unequivocally supported statins for primary prevention. In one of the studies, a 9% decrement was noted in all-cause mortality and a 25% lowering of major vascular events with every mmol/L reduction of LDL-C level, even among low-risk asymptomatic patients [23]. The finding of this study was supported by another recent systematic review in which a 14% decrement in all-cause mortality and a 25% lowering of CVD events were reported with statin use [20].
In 2013, Minder et al. [24] reported that statins effectively lowered LDL, very-low-density lipoprotein, and intermediate-density lipoproteins cholesterol particles, resulting in clinically significant lowering of cardiovascular deaths/disabilities. From these findings, it was concluded that primary prevention with statins in cardiovascular benefits outweigh its adverse effects [24]. There remains palpable doubt in the mind of clinicians over the prescription of statins, as consensus cannot be built on the distinction of low-risk, moderate-risk, or high lifetime risk of CVD among patients. Further, it is not possible to unequivocally establish whether those at low-risk or moderate-risk have benefited from primary prevention with statins. It has therefore been suggested that statin use should either be individualized for the low-risk group or be based on clinical judgment in order to reduce undue lifelong statin prescription to apparently healthy individuals and considerably lower health costs for individuals [6].
Clinical evidence for primary prevention with statins
Patients have a substantial risk for future cardiovascular events and premature death if they manifest CHD or show other clinical signs of atherosclerosis or diabetes. Hypolipidemic therapy is regarded as the therapy of choice in the prevention of CVD, and use of this therapy has largely outweighed the risk associated with disease treatment [25,26].
In 1995, Shepherd et al. [27] investigated primary prevention with pravastatin in a clinical study that involved 6595 patients with hypercholesterolemia and with a relatively low risk of CVD. Pravastatin, at a dose of 40 mg, demonstrated a 32% decrement in the risk of major cardiovascular events over a follow-up period of 4.9 years. In 1997, Down et al. [28] confirmed the efficacy and safety of another important statin, lovastatin, in healthy human subjects (lower-risk groups). This study showed that lovastatin reduced the occurrence of major coronary events by 39%, confirming the benefits of statin treatment among healthy human subjects [28,29].
In a 2002 clinical study involving 1585 subjects taking pravastatin, Shepherd and coworkers [30] reported a higher risk of cancer and other side effects among these subjects. However, later several studies [30–35] demonstrated statins’ positive association with low risk of coronary and other CVD-related disorders, no increased risk of cancer, and provided the critical evidence for statin use in the primary prevention of diabetes. The National Institute for Health and Care Excellence in the United Kingdom proposed a threshold of CHD risk of ⩾20% over 10 years for the introduction of statins for primary prevention in patients with type 2 diabetes [36]. In 2006, Nakamura et al. [37] conducted a clinical study that favored primary prevention with statin therapy. This study involved 3966 Japanese women with increased blood cholesterol concentration. Study participants were randomly allocated in two groups, which included diet and diet plus pravastatin (10–20 mg), and followed-up over a period of 5.3 years. The study results showed that pravastatin reduced the risk of coronary events by 23% and without any significant difference in the risk of cancer or other side-effects between the two groups.
In 2010, Chan and colleagues [38] reported on a trial that assessed the intensive lipid lowering effects of rosuvastatin on the progression of aortic stenosis in asymptomatic patients with mild to moderate aortic stenosis. In this trial, however, a 40 mg dose of rosuvastatin failed to reduce the rate of progression of aortic stenosis in patients with mild to moderate aortic stenosis.
In 2008, in a major landmark intervention trial evaluating rosuvastatin, Hlatky [39] highlighted the advantages of primary prevention with statins. The trial was a double-blind, placebo-controlled, multicenter, randomized trial conducted at more than thousand sites across 26 countries and was financially sponsored by pharmaceutical giant AstraZeneca. It investigated 20 mg daily dose of rosuvastatin in decreasing the rate of occurrence of first major cardiovascular events-nonfatal MI, stroke (non-fatal), unstable angina (requiring hospitalization), arterial revascularization, and mortality from cardiovascular events in comparison to placebo [39,40]. This trial potentially changed the spectrum of statin therapy outward to include even more of the general population. The trial recruited around 18,000 healthy subjects with no known CVD or diabetes, LDL-C level less than 130 mg/dL and a high sensitive C-reactive proteins (CRP) of ⩾2 mg/L. Rosuvastatin decreased cardiovascular events in patients with both elevated CRP and LDL <130 mg/dL, but otherwise healthy. In the rosuvastatin group in this trial, a lower proportion (0.9%) of patients reported major cardiac events in comparison to the placebo group (1.8%) indicating large absolute benefits of treatment with statins, enough to justify the associated risk and costs [39]. The study also revealed a significant lowering of cardiovascular events across population, such as women, black and Hispanic populations, for which primary prevention data were limited. The findings of this trial led to a revision in primary prevention guidelines. A summary of major and other primary prevention trials with statins are summarized in Table 1.
Table 1.
Summary of major primary prevention trials with statins.
| S. No. | Trials | Trial period/follow up | Drugs | N | Primary endpoint | Secondary endpoints | Results |
|---|---|---|---|---|---|---|---|
| 1. | WOSCOPS | Follow up 4.9 year | Pravastatin, 40 mg every evening | 6595 | Occurrence of nonfatal MI or CHD death as first event | Nonfatal MI or CHD death | Pravastatin treatment in hypercholesterolemic patients with symptomatic CHD, lowers LDL-C, reduces fatal and nonfatal CVE rates |
| 2. | AFCAPS/TexCAPS | Follow up 5.2 year | Lovastatin (20–40 mg/d) | 6605 (5608 men and 997 women) | First acute major coronary event | Fatal or nonfatal revascularization; Fatal or nonfatal MI, UA; CV mortality; CHD deaths | First acute major coronary event reduced by 37%, MI by 40%, Unstable angina by 32%, and coronary revascularization by 33% |
| 3. | ALLHAT-LLT | Follow-up of 3.3 year | Pravastatin, (20 and 40 mg) | 10,355 | All-cause mortality; fatal coronary heart disease and nonfatal MI | Fatal CHD and nonfatal MI, stroke, CHF, cancer | There was no difference in mortality, CHD or stroke compared with usual care for moderate hypercholesterolemia |
| 4. | ASCOT-LLA | Mean follow up 4.8 years | Atorvastatin (10 mg) | 10,305 | Nonfatal MI and fatal CHD | Coronary events; all cause mortality; fatal/nonfatal stroke | Primary endpoint was significantly reduced (1.9% versus 3.0%); insignificant reduction in all cause mortality but trended towards reduction; total cholesterol reduced by 24% at 12 months and 19% after 3 years compared with placebo |
| 5. | CARDS | 4 years | Atorvastatin (10 mg/d or placebo) | 2838 | First occurrence of acute coronary events, coronary revascularization or stroke | Total mortality, any CV endpoints, lipids and lipoproteins | Reduction in major CV events by 37% |
| 6. | ASPEN | Follow up 2.4 years | Atorvastatin 10 mg | 2410 | First occurrence of composite clinical endpoints of CV death, MI, stroke, recanalization, worsening angina | Insignificant reduction in the primary composite end point comparing 10 mg of atorvastatin with placebo (13.7 and 15.0%) | |
| 7. | MEGA | Mean follow-up 5.3 years | Pravastatin (10–20 mg/d) | 7832 | First occurrence of CHD | Reduction of CHD risk by 33% compared with diet alone | |
| 8. | JUPITER | Median follow up 1.9 years (trail halted); maximal follow up 5 years | Rosuvastatin (20 mg/d) | 17,802 | First major CV (nonfatal MI, nonfatal stroke, hospitalization for UA, arterial revascularization procedures and confirmed CV deaths | Components of primary endpoints considered individually | 44% reduction in primary end point of all vascular events, 54% reduction in MI, 48% reduction in stroke, 46% reduction in need for arterial revascularization, and 20% reduction in all cause mortality. (trial halted on the recommendation of independent data and safety monitoring board) |
CHF = congestive heart failure; CHD = coronary heart disease; CV = cardiovascular; MI = myocardial ischemia; UA = unstable angina; N = No. of patients. AFCAPS/TeXCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study Air Force/Texas Coronary Atherosclerosis Prevention Study; ALLHAT–LLT = Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack–Lipid Lowering Trial; ASCOT–LLA = Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm; ASPEN = Atorvastatin for Prevention of Coronary Heart Disease Endpoints in Non-insulin Dependent Diabetes Mellitus; CARDS = Collaborative Atorvastatin Diabetes Study; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; MEGA = Management of Elevated Cholesterol in Primary Prevention of Adult Japanese; WOSCOPS = West of Scotland Coronary Prevention Study.
Primary prevention in men and women aged ⩾21 years with LDL-C ⩾190 mg/dL
Individuals ⩾21 years of age with primary and high LDL-C (⩾190 mg/dL) are placed in the highly susceptible category to developing atherosclerotic-CVD (ASCVD) events during their lifetime due to genetic predisposition. These individuals have a greater risk of developing increased LDL-C levels. Therefore, it was recommended that at age 21, individuals having genetic predisposition to CVD should be prophylactically treated with statins even if they were not treated before or had no prior diagnosis. Treatment with statins was reported to decrease over 20% risk of ASCVD with every 39 mg/dL decrease in LDL-C. It was reported that individuals with LDL-C ⩾190 mg/dL would need even further reduction in LDL-C levels and hence, such individuals should be managed intensively for other risk factors to reduce the risk of ASCVD events. Therefore, the new guidelines justified reducing LDL-C level to at least half with high-intensity statin therapy. However, high dose statin alone cannot lower LDL-C adequately to reduce the risk of ASCVD events in individuals with such a high LDL-C level. Addition of non-statin cholesterol-lowering medications may be required besides prescribing a maximal tolerated statin dose to attain desired LDL-C targets in such individuals [40].
Primary prevention in patients with diabetes
Strong evidence supports treatment of diabetic patients aged 40–75 years with a moderate-intensity statin dose. In diabetic patients, only one trial of high-intensity statin therapy in primary prevention was conducted but high level of evidence was taken into consideration for cardiovascular events with statin therapy reduction in individuals with >7.5% estimated 10-year ASCVD risk and who were non diabetic to recommend high-intensity statin therapy than individuals with diabetes and >7.5% estimated 10-year ASCVD risk. Individuals with diabetes experience greater morbidity and have worse rates of survival following the onset of clinical ASCVD. Therefore, personalization of statin therapy was recommended in individuals with diabetes who are <40 and >75 years of age considering ASCVD risk reduction benefits, the potential for adverse effects and drug-drug interactions, and patient preferences [41,42].
Primary prevention in non-diabetic patients and patients with LDL-C 70–189 mg/dL
Recent guidelines have recommended statin therapy based on an estimated 10-year ASCVD risk, regardless of sex, race or ethnicity, and in individuals aged 40–75 years with LDL-C levels between 70 and 189 mg/dL who were without clinical ASCVD or diabetes. Point estimates of statin-associated reductions in the relative risk of ASCVD in primary prevention reveal a similar reduction of ASCVD risk in both men and women. There was also no evidence that the ASCVD risk-reduction benefit or adverse-effect profiles differed by race. Statin therapy was recommended to individuals with ⩾7.5% estimated 10-year risk for ASCVD and LDL-C 70 to189 mg/dL in spite of the fact that these individuals aged 40–75 years were not prior candidates for statin therapy based on the presence of clinical ASCVD, diabetes, or LDL-C ⩾190 mg/dL. It was reported that only one exclusively primary prevention randomized clinical trial had included individuals with LDL-C 70 to <100 mg/dL. However, the Cholesterol Treatment Trialists Collaboration 2010 meta-analysis found a relative reduction in ASCVD events of similar magnitude across the spectrum of LDL-C levels >70 mg/dL [41,42].
Primary prevention with statins in CVD in past and present treatment guidelines
In some way or another, several guidelines have advocated the use of statins. However, there remains a lack of consensus among the global medical community on the primary prevention of CVD with statins.
The National Cholesterol Education Program Adult Treatment Panel (NCEP-ATP) III guidelines recommend the primary prevention with statins for LDL-C level ⩾190 mg/dL (∼5.0 mmol/L) and advise on the use of clinical judgment for LDL-C between 160 and 189 mg/dL (∼4.1–4.9 mmol/L). They do not recommend statin use in apparently healthy subjects with an LDL-C <160 mg/dL (∼4.1 mmol/L), unless an individual presents with two or more CVD risk factors [16]. Statins were recommended as the drug of choice for lowering high blood cholesterol in European guidelines such as the European Society on Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines for the management of dyslipidemia, as well as in the revised version of the ESC/EAS guidelines on CVD prevention. The ESC/EAS guidelines arbitrarily divided asymptomatic population into two groups based on particular LDL-C cutoffs. The first group included individuals at high risk based on Systematic Coronary Risk Estimation (SCORE) > 5%, and in whom preventive action should be maximized. This was misinterpreted by many physicians (sympathizers of the pharmaceutical manufacturers) that everyone in this group should take lipid-lowering drugs. The second group included those with SCORE < 5%. Individuals in this group were not recommended preventive action nor prescribed drugs. In 2012, a revised version of the European guidelines on CVD prevention was published. The revised guidelines deemed wrong the prescription of statin to individuals falling in the above two categories, which are quite prevalent in clinical practices [43,44]. The revised ESC/EAS guidelines also had the demerit in that they recommended statin use based on CVD-risk estimation as per SCORE charts, and did not use any target LDL-C levels. Total CVD-risk is known to be intricately linked to a multitude of predisposing factors. Therefore, in the revised ESC/EAS guidelines, cutoffs defining ‘high-risk group’ are random and based on risk-levels at which patients show benefit in clinical studies [45,46]. Therefore, ESC/EAS guidelines advocate the adoption of an individualized approach to statin prescription [45].
The recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines, released in November 2013, recommended a more aggressive use of statin, and emphasized the need to prevent stroke and CVDs by focusing on statin therapy instead of achieving LDL-C targets using pharmacotherapeutic agents [42]. Accordingly, as per the new ACC/AHA guidelines, an exhaustive statin therapy is superior to a less exhaustive statin therapy for patients. The new ACC/AHA guidelines showed that individuals with prior vascular disease or LDL-C = 4.9 mmol/L [190 mg/dL] should be considered for high-intensity statin therapy to achieve at least 50% lowering of LDL-C. The advantages of statin use on heart attack, stroke, and cardiovascular mortality considerably outweighed the risk of myopathy or diabetes. These guidelines used a recently developed risk prediction matrix based on “hard” atherosclerotic events to suggest statin therapy for primary prevention of patients with a predicted 10-year risk ⩾7.5%, and considered statin therapy in individuals with 10-year risks of between 5% and 7%. In type 1 or type 2 diabetic patients, the threshold of ⩾7.5% was used to make decisions on using high-intensity or moderate-intensity regimen with statins, and was defined as a daily regimen of statin that lowered LDL-C by >50% or between 30% and 50%, respectively. According to the criteria of the recent AHA/ACC, over 45 million middle-aged Americans without CVD are eligible for treatment with statins, and with the new risk calculator, almost all men in the age bracket of >66 years and women >70 have a 10-year risk greater than 7.5% possessing favorable risk factors that would require treatment with statins. Therefore, age alone could be used to screen individuals for possible treatment with statin therapy. With this approach, the new ACC/AHA guidelines propose to simplify and improve care for individuals with higher CVD risks, including those with diabetes [42]. However, the potential flaw in the recent ACC/AHA guidelines are that they rely on the new risk prediction algorithm which could justify primary prevention with statin in many for whom trial evidence is lacking. Further, it could put many out of the ambit of statin use despite clinical trial evidence suggesting use of statin. Therefore, the recent ACC/AHA guidelines urge clinicians to adopt an alternative and simpler policy of assessing “what works best” and “in whom” than in predicting risk and presuming benefits in primary prevention with statin based on the available clinical trial data. This would minimize undue statin prescription and would help make evidence-based clinical recommendations for statin therapy backed by clinical data [22]. The recent ACC/AHA guidelines justify the cost of generic statin medication as there is an overall reduction in cardiovascular morbidity following primary prevention with statin therapy. Primary prevention with statin resulted in a 29% decrease in hospitalization rates and an average reduction of 27% in overall healthcare costs indicating cost effectiveness of statin therapy in primary prevention [25].
Discussion
In Saudi Arabia, coronary heart disease has been one of the main health hazards and the third most common cause of hospitalization and mortality, followed by accident and senility. A surge in CVD-related morbidity and mortality is natural because of the large epidemiologic and nutritional transition in the kingdom. Saudi Arabia has also witnessed considerable economic growth, standard of living and life-style transformations, including adoption of sedentary lifestyle and access to higher energy diet intake, and increased urbanization. These transitions have led to the emergence of the epidemic of noncommunicable diseases, and hence an increase in the number of CVD-related deaths and disabilities. It was reported that nations witnessing transitions from conventional semi-urbanized lifestyles to western lifestyles at a fast pace were susceptible to an increase in populations with obesity and other cardiovascular problems [47]. In 2004, Al-Nozha et al. reported that the overall prevalence of coronary artery diseases in Saudi Arabia was 5.5%, a figure that was midway between those reported from other countries [48]. High dietary fat intake, hypertension, diabetes mellitus, smoking, and lack of physical exercise have been commonly observed risk factors among Saudi citizens with heart diseases [49]. It is strange that by the time heart problems are detected, the underlying cause (atherosclerosis) has progressed to an advanced stage [50]. Twenty years ago, a study from the Eastern Province of Saudi Arabia reported that more than 26% of total deaths were from CVD alone [51]. The prevalence study reported that as the population grew with time, CVD-related morbidity and mortality also rose several times. The population of Saudis aged 60 or more has grown several folds over the last 20 years, and these elderly people are vulnerable to various types of heart diseases. In 2004, Al-Shehri et al. [52] reported the presence of high-risk levels of total cholesterol, LDL-C and triglycerides in 32.7%, 33.1%, and 34.1%, respectively, among school children aged 9–12 years. In 2008, Al-Nozha et al. [53] conducted a study over a five-year period between 1995 and 2000 with selected Saudis in the age group of 30–70 years, and showed that the prevalence of hypercholesterolemia among males was 54.9% and 53.2% for females, and 53.4% among urban Saudis and 55.3% among rural Saudis. Males were reported to have a statistically significant higher prevalence of hypertriglyceridemia over 47.6% compared to 33.7% in females [53].
In a study of students, conducted at the King Faisal University, Dammam, Saudi Arabia, the presence of CVD risk factors was high, and a quarter of the students reported not practicing physical exercise at all, while 18.9% of them were smokers. The study also reported that a high proportion of university students consumed fast foods, saturated fats, and soft drinks. Overweight and obesity as measured by body mass index (kilograms/square of the height in meters) and unacceptable waist-to-hip ratios were evident in Saudi students [49]. Obesity as measured by waist-to-hip ratio is described as a better tool in the prediction of CVD and mortalities related to other heart diseases [54]. Several local studies have also reported an increased prevalence of diabetes mellitus, smoking, obesity and hypercholesterolemia. In a 2012 study of 4490 Saudis aged 15 years and older Al-Kaabba et al. [55] reported high prevalence of dyslipidemia in Saudi Arabia. In 2013, Basulaiman et al. [56] conducted an epidemiological study involving face-to-face interviews, with a national multistage representative sample of Saudis aged 15 years or older (10,735 participants) between April and June 2013. The study reported that, overall, 8.5% of Saudis were hypercholesterolemic, and another 19.6% were borderline hypercholesterolemic. Of the hypercholesterolemic Saudis, 65.1% were undiagnosed, 2.3% were treated uncontrolled, 28.3% were treated controlled, and 4.3% were untreated. The risk of being hypercholesterolemic increased with age and among individuals who reported consuming margarine, obese individuals, and those who had been previously diagnosed with hypertension or diabetes [56]. The study concluded that more than a million Saudis were hypercholesterolemic and 0.7 million of them were unaware of their condition, which could be controlled through early detection. In a recent cross section study, involving medical students of King Abdulaziz University, an alarmingly high prevalence of CVD risk factors were reported among medical students, especially among males [57]. The risk of mortality for patients with heart diseases is well-known and a number of guidelines exist to reduce this risk; yet, hypercholesterolemia among patients in the six Arabian Gulf countries including Saudi Arabia is still undertreated.
In light of such studies reporting higher prevalence rates of hypercholesterolemia and CVD-risk factors in Saudi Arabia, there is an urgent call for the aggressive management of dyslipidemia and associated risk factors. Several guidelines are currently available for the management of dyslipidemia and CVD-risk factors, which now include the new ACC/AHA guidelines. However, implementation of appropriate lipid management guidelines would be an important step forward in containing rising incidences of CVDs in the Kingdom of Saudi Arabia. In a recent review, published in 2014, Ray and colleagues conduct a comparison of the widely used 2011 ESC/EAS guidelines and the new 2013 ACC/AHA guidelines [58]. Both the 2011 ESC/EAS and the 2013 ACC/AHA guidelines have used overall cardiovascular risk to assess any imminent CVD risk and to make clinical decisions on whether or not to prescribe lipid-modifying agent. However, the new ACC/AHA guidelines focus primarily on statins only because these are the most widely prescribed lipid-modifying drugs to date and whose benefits in the prevention of CVD have been unambiguously established. Hence, reports suggest that the new 2013 ACC/AHA guidelines have a limited scope as they only consider evidence from RCTs and ignore broad scientific evidence [58]. The 2011 ESC/EAS guidelines have a considerably greater scope as these consider all the available evidence and not just clinical evidence from randomized trials. Besides, 2011 ESC/EAS guidelines stressed on the importance of all lipids and provides practical guidance across a wide range of conditions including ASCVD prevention and dyslipidemia. The 2013 ACC/AHA guidelines recommended high-intensity (high dose) and moderate-intensity (moderate dose) statins without regard to the risk of new-onset diabetes, myopathy and hemorrhagic shocks [42]. They recommended the statin doses that were used in trials, and pushed for the achievement of a 50% reduction in LDL-C using high-intensity statins (atorvastatin 40/80 mg or rosuvastatin 20 mg), and a 30–50% reduction using moderate intensity statins (atorvastatin 10 mg, simvastatin 20 or 40 mg, pravastatin 40 mg, fluvastatin 40 mg, twice daily, and rosuvastatin 10 mg). However, it is worthy to note here that rosuvastatin 40 mg as high-intensity statin, and atorvastatin 20 mg and rosuvastatin 5 mg doses are not RCT tested but are doses approved by the Food and Drug Administration, USA. There is no hard RCT evidence suggesting 50% reduction of LDL-C and therefore it would be incorrect to make it the only criteria in the recommendation of statins. The new ACC/AHA guidelines are in complete contradiction with the existing NCEP-ATP III and other international guidelines, all of which all have specify LDL-C goals. Further, the 2013 ACC/AHA guidelines were unable to explain the role of additional lipid-lowering therapies in individuals who, regardless of achieving 50% reduction in LDL-C, show high residual absolute-risk. The 2011 ESC/EAS guidelines have recommended specific LDL-C targets for each level of absolute risk as they categorize people into moderate and very high CVD risks. Moreover, the 2011 ESC/EAS guidelines recommend LDL-C and other lipid measures to monitor efficacy, compliance, and residual risk, and leave considerable scope to individualized patient-care and consideration of additional therapies as per the clinical situation [44,45]. The further reported weakness in the 2013 ACC/AHA guidelines was that they offered guidance only on treating those at risk of ASCVD and did not consider lipid fractions as potential markers of CVD, other than LDL-C [42]. On the other hand, the 2011 ESC/EAS guidelines provided a systematic approach in the treatment of CVD risk and broader understanding of LDL-C in CVD risk assessment. They also placed emphasis on the monitoring of LDL-C for measuring therapeutic efficacy and patient compliance. The advantage of the 2011 ESC/EAS guidelines was that they provided a realistic clinical solution for the utility of other lipid fractions that the new ACC/AHA guidelines ignored, such as triglycerides-rich lipoproteins, remnants, and conditions associated with low HDL-C, non-HDL-C or apolipoprotein B. These lipid fractions are reported as informative and can help in clinical decision-making. In addition to the traditional-risk factors, ESC/EAS guidelines also recognized other factors which included elevated social deprivation, central obesity, triglycerides, elevated lipoprotein (a), subclinical atherosclerosis, or family history of premature CVD which may further influence absolute risk. In contrast, very high HDL-C or family history of longevity was also part of the consultation with the patient before prescribing lipid-modifying therapy. Both guidelines, however, were unanimous in their suggestions for lifestyle modifications and emphases on making patients partners in disease prevention, and both underscored the importance of clinical judgment over guidelines in individual cases.
In primary prevention, the new ACC/AHA guidelines recommend that statin treatment should be initiated in individuals with a 10-year ASCVD risk of ⩾7.5% compared to previous recommendations prescribing a considerably higher threshold for 10-year risk of fatal and non-fatal coronary heart disease. In the SCORE model, the ACC/AHA 10-year threshold of 7.5% is equivalent to a 2.5% risk for CVD death over 10 years, and individuals with a 10-year risk of fatal CVD of 2.5% are regarded as at moderate risk. In that situation, the ESC/EAS recommends the LDL-C goal of 3 mmol/L. The 2013 ACC/AHA-prescribed threshold for primary prevention, if applied, would complicate healthcare by increasing multifold the number of people receiving statins over the number of people who should receive statin doses, and who in many cases may end up receiving higher doses than prescribed. In turn, this would considerably raise healthcare costs. Similarly, as more people receive moderate to high-intensity statin doses, there would be a manifold increase in statin side-effects complaints and/or the development of statin intolerance. Therefore, the practicality of extending statin use to an even wider group is worthy of reconsideration, even if the benefits with statins across a range of clinical conditions is unequivocally established. Almost all the world guidelines, including Asia, Australia, and Canada, have given due consideration to evidence beyond RCTs and prescribe lipid goals to monitor the response to lipid modification therapy and compliance of patient. Hence, lipid management guidelines from the rest of the world resemble the 2011 ESC/EAS guideline and not the new ACC/AHA guidelines. The guidelines used in most parts of the world are designed to provide a holistic lipid-management solution that could be applied to a large patient-population. Furthermore, the ACC/AHA mixed pooled cohorts equation cannot be applied globally as patients from other countries, such as south-east Asia, the Indian subcontinent, Pacific islanders including Maori and Australian aboriginals, were not included in the clinical trials. In some ethnic groups, a higher number of statin side-effects has been reported. According to Ray et al., the 2013 ACC/AHA guidelines are impractical in the Asia–Pacific region [58]. The comparison of the new ACC/AHA guidelines vis-à-vis the 2011 ESC/EAS guidelines indicate that it would not be appropriate to draw primary prevention guidance from the new ACC/AHA guidelines which is riddled with fallacies. The pattern of CVD-risk factors prevalent in Saudi Arabia are mostly observed as lifestyle-related which include smoking, lack of exercise, and low fibrous diets. Implementing the new ACC/AHA guidelines would lead to the inclusion of a large population for statin therapy, to an increase in the number of people with statin intolerance and an increase in myopathy, diabetes mellitus, hepatic impairment, rash and flushing, and neurological disorders. It would be more practical to adopt and seek guidance from the 2011 EAS/ESC guidelines, which provide a comprehensive approach to dyslipidaemia management for primary prevention with statins in the Kingdom of Saudi Arabia.
Conclusion
There is a prevalence of lipid-related disorders in Saudi Arabia which mostly results from lifestyle changes. It is preferable to prescribe statin primary prevention to a select few in order to avoid overmedicating the general population. Since the release of the new ACC/AHA guidelines, there has been an ever increasing worldwide prevalence of CVD and a confusion has prevailed in many regions which do not have their own guidelines. The reduction of primary prevention threshold, as prescribed by the new ACC/AHA guidelines may benefit younger patients with high absolute lifetime risk, but may lead to over-treatment of older patients, due to the overemphasis on age. The new ACC/AHA guidelines are riddled with fallacies and a comparison vis-à-vis ESC/EAS guidelines emphasize that its general adoption could result in the unnecessary prescription of statins to a large number of patients, potentially at considerable cost. Therefore, considering the prevailing clinical data on CVD in Saudi Arabia, the 2011 ESC/EAS guidelines for lipid modification appear to be the most wide-ranging, realistic, and appropriate option.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Mendis S., Lindholm L.H., Anderson S.G., Alwan A., Koju R., Onwubere B.J. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol. 2011;64(12):1451–1462. doi: 10.1016/j.jclinepi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ramahi T.M. Cardiovascular disease in the Asia Middle East region: global trends and local implications. Asia Pac J Public Health. 2010;22(3 Suppl.):83S–89S. doi: 10.1177/1010539510373034. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization: Non Communicable Disease Country Profiles (Saud Arabia), 2011. <http://www.who.int/nmh/countries/sau_en.pdf> [accessed 29.05.14].
- 4.22% deaths in KSA due to heart disease, stroke. Saudi Gazette. 2009 January 15. <http://www.saudigazette.com.sa/index.cfm?method=home.regcon&contentid=2009011526753> [accessed 6.03.14].
- 5.Alamoudi O.S., Attar S.M., Ghabrah T.M., Al-Qassimi M.A. Pattern of common diseases in hospitalized patients at an University Hospital in Saudi Arabia; a study of 5594 patients. J King Abdulaziz Univ Med Sci. 2009;16(4):3–12. [Google Scholar]
- 6.Reiner Z. Statin in the primary prevention of cardiovascular disease. Nat Rev Cardiol. 2013;10:453–464. doi: 10.1038/nrcardio.2013.80. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q., Liao J.K. Rho kinase: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des. 2009;15(27):3108–3115. doi: 10.2174/138161209789057986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Aelst L., D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11(18):2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 9.Weitz-Schmidt G., Welzenbach K., Brinkmann V., Kamata T., Kallen J., Bruns C. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001;7(6):687–692. doi: 10.1038/89058. [DOI] [PubMed] [Google Scholar]
- 10.Shishehbor M.H., Aviles R.J., Brennan M.L., Fu X., Goormastic M., Pearce G.L. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA. 2003;289(13):1675–1680. doi: 10.1001/jama.289.13.1675. [DOI] [PubMed] [Google Scholar]
- 11.Tandon V., Bano G., Khajuria V., Parihar A., Gupta S. Pleiotropic effects of statins. Indian J Pharmcol. 2005;37(2):77–85. [Google Scholar]
- 12.Aikawa M., Rabkin E., Sugiyama S., Voglic S.J., Fukumoto Y., Furukawa Y. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103(2):276–283. doi: 10.1161/01.cir.103.2.276. [DOI] [PubMed] [Google Scholar]
- 13.Liu P.Y., Liu Y.W., Lin L.J., Chen J.H., Liao J.K. Evidence for statin pleiotropy in humans: differential effects of statins and ezetimibe on rho-associated coiled-coil containing protein kinase activity, endothelial function, and inflammation. Circulation. 2009;119(1):131–138. doi: 10.1161/CIRCULATIONAHA.108.813311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy S.M., Cleeman J.I., Merz C.N., Brewer H.B., Jr, Clark L.T., Hunninghake D.B. A summary of implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Arterioscler Thromb Vasc Biol. 2004;24(8):1329–1330. doi: 10.1161/01.ATV.0000139012.45265.e0. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C., Keech A., Kearney P.M., Blackwell L., Buck G., Pollicino C. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 16.Brugts J.J., Yetgin T., Hoeks S.E., Gotto A.M., Shepherd J., Westendorp R.G. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106(25):3143–3421. [PubMed]
- 18.Greving J.P., Visseren F.L., DeWit G.A., Algra A. A. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ. 2011;342:d1672. doi: 10.1136/bmj.d1672. [DOI] [PubMed] [Google Scholar]
- 19.Taylor F., Ward K., Moore T.H., Burke M., Davey Smith G., Casas J.P. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2011;1:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor F., Huffman M.D., Macedo A.F., Moore T.H., Burke M., Davey Smith G. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:CD004816. doi: 10.1002/14651858.CD004816.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Vries F.M., Denig P., Pouwels K.B., Postma M.J., Hak E. Primary prevention of major cardiovascular and cerebrovascular events with statins in diabetic patients: a meta-analysis. Drugs. 2012;72(18):2365–2373. doi: 10.2165/11638240-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Minder C.M., Blaha M.J., Horne A., Michos E.D., Kaul S., Blumenthal R.S. Evidence-based use of statins for primary prevention of cardiovascular disease. Am J Med. 2012;125(5):440–446. doi: 10.1016/j.amjmed.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Cholesterol Treatment Trialists’ (CTT) Collaborators, Mihaylova B., Emberson J., Blackwell L. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minder C.M., Blumenthal R.S., Blaha M.J. Statins for primary prevention of cardiovascular disease: the benefits outweigh the risks. Curr Opin Cardiol. 2013;28(5):554–560. doi: 10.1097/HCO.0b013e32836429e6. [DOI] [PubMed] [Google Scholar]
- 25.Ridker P.M., Cook N.R. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382(9907):1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 26.Cheung B.M., Lam K.S. Is intensive LDL-cholesterol lowering beneficial and safe? Lancet. 2010;376(9753):1622–1624. doi: 10.1016/S0140-6736(10)61545-0. [DOI] [PubMed] [Google Scholar]
- 27.Shepherd J., Cobbe S.M., Ford I., Isles C.G., Lorimer A.R., MacFarlane P.W. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 28.Downs J.R., Beere P.A., Whitney E., Clearfield M., Weis S., Rochen J. Design & rationale of the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS) Am J Cardiol. 1997;80(3):287–293. doi: 10.1016/s0002-9149(97)00347-0. [DOI] [PubMed] [Google Scholar]
- 29.Downs J.R., Clearfield M., Weis S., Whitney E., Shapiro D.R., Beere P.A. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615–1622. doi: 10.1001/jama.279.20.1615. [DOI] [PubMed] [Google Scholar]
- 30.Shepherd J., Blauw G.J., Murphy M.B., Bollen E.L., Buckley B.M., Cobbe S.M. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 31.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002;288(23):2998–3007. [DOI] [PubMed]
- 32.Sever P.S., Dahlöf B., Poulter N.R., Wedel H., Beevers G., Caulfield M. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–1158. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 33.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360(9326):7–22. [DOI] [PubMed]
- 34.Colhoun H.M., Betteridge D.J., Durrington P.N., Hitman G.A., Neil H.A., Livingstone S.J. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 35.Knopp R.H., d’Emden M., Smilde J.G., Pocock S.J. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diab Care. 2006;29(7):1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence (NICE), 2006. Statins for the prevention of cardiovascular events. www.nice.org.uk/nicemedia/pdf/TA094guidance.pdf.
- 37.Nakamura H., Arakawa K., Itakura H., Kitabatake A., Goto Y., Toyota T. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–1163. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 38.Chan S.H., Wu K.L., Kung P.S., Chan J.Y. Oral intake of rosiglitazone promotes a central antihypertensive effect via upregulation of peroxisome proliferator-activated receptor-gamma and alleviation of oxidative stress in rostral ventrolateral medulla of spontaneously hypertensive rats. Hypertension. 2010;55(6):1444–1453. doi: 10.1161/HYPERTENSIONAHA.109.149146. [DOI] [PubMed] [Google Scholar]
- 39.Hlatky M.A. Expanding the orbit of primary prevention–moving beyond JUPITER. N Engl J Med. 2008;359(21):2280–2282. doi: 10.1056/NEJMe0808320. [DOI] [PubMed] [Google Scholar]
- 40.Ridker P.M., Danielson E., Fonseca F.A., Genest J., Gotto A.M., Jr, Kastelein J.J. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 41.Ridker P.M. JUPITER Study Group. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: rationale and design of the JUPITER trial. Circulation. 2003;108(19):2292–2297. doi: 10.1161/01.CIR.0000100688.17280.E6. [DOI] [PubMed] [Google Scholar]
- 42.Stone NJ, Robinson JG, Lichtenstein AH, Goff DC Jr, Lloyd-Jones DM, Smith SC Jr, et al. ACC/AHA cholesterol guideline panel. Treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task force on Practice guidelines. Circulation 2014;129(25 Suppl 2):S1–45. [DOI] [PubMed]
- 43.Graham I., Atar D., Borch-Johnsen K., Boysen G., Burell G., Cifkova R. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2007;28(19):2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- 44.Reiner Z. New ESC/EAS guidelines for the management of dyslipidaemias – any controversies behind the consensus? Eur J Cardiovasc Prev Rehabil. 2011;18(5):724–727. doi: 10.1177/1741826711418946. [DOI] [PubMed] [Google Scholar]
- 45.European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32(14):1769–1818. [DOI] [PubMed]
- 46.Perk J., De Backer G., Gohlke H., Graham I., Reiner Z., Verschuren M. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The fifth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur Heart J. 2012;33(13):1635–1701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 47.Boumedjout H. Cardiovascular diseases on the increase in Arab states. Nature Middle East. doi:10.1038/nmiddleeast.2012.36.
- 48.Al-Nozha M.M., Arafah M.R., Al-Mazrou Y.Y., Al-Maatouq M.A., Khan N.B., Khalil M.Z. Coronary artery disease in Saudi Arabia. Saudi Med J. 2004;25(9):1165–1171. [PubMed] [Google Scholar]
- 49.Sabra A.A., Taha A.Z., Al-Sebiany A.M., Al-Kurashi N.Y., Al-Zubier A.G. Coronary heart disease risk factors: prevalence and behavior among male university students in Dammam City, Saudi Arabia. J Egypt Public Health Assoc. 2007;82(1–2):21–42. [PubMed] [Google Scholar]
- 50.Kumosani T.A., Alama M.N., Iyer A. Cardiovascular diseases in Saudi Arabia. Prime Res Med. 2011;1:1–6. [Google Scholar]
- 51.Alobaid A.A., Gilchrist R., Bointon B. Coronary heart disease mortality in the eastern province of Saudi Arabia in 1989 and 1990. Ann Saudi Med. 1994;14(5):387–391. doi: 10.5144/0256-4947.1994.387. [DOI] [PubMed] [Google Scholar]
- 52.Al-Shehri S.N., Saleh Z.A., Salama M.M., Hassan Y.M. Prevalence of hyperlipidemia among Saudi school children in Riyadh. Ann Saudi Med. 2004;24(1):6–8. doi: 10.5144/0256-4947.2004.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Nozha M.M., Arafah M.R., Al-Maatouq M.A., Khalil M.Z., Khan N.B., Al-Marzouki K. Hyperlipidemia in Saudi Arabia. Saudi Med J. 2008;29(2):282–287. [PubMed] [Google Scholar]
- 54.Welborn T.A., Dhaliwal S.S., Bennett S.A. Waist-hip ratio is the dominant risk factor predicting cardiovascular death in Australia. Med J Aust. 2003;179(11–12):580–585. doi: 10.5694/j.1326-5377.2003.tb05704.x. [DOI] [PubMed] [Google Scholar]
- 55.Al-Kaabba A.F., Al-Hamdan N.A., Tahir A.E., Abdalla A.M., Saeed A.A., Hamza M.A. Prevalence and correlates of dyslipidemia among adults in Saudi Arabia: results from a national survey. Open J Endocr Metab Dis. 2012;2:89–97. [Google Scholar]
- 56.Basulaiman M, Bcheraoui CE, Memish ZA, Tuffaha M, Daoud F, Robinson M, et al. Hypertension and its associated risk factors-Kingdom of Saudi Arabia. Ann Epidemiol 2014, pii: S1047-2797(14)00375-5. http://dx.doi.org/10.1016/j.annepidem.2014.08.001.
- 57.Ibrahim NK, Mahnashi M, Al-Dhaheri A, Al-Zahrani B, Al-Wadie E, Aljabri M, et al. Risk factors of coronary heart disease among medical students in King Abdulaziz University, Jeddah, Saudi Arabia. BMC Public Health 2014;14:411. [DOI] [PMC free article] [PubMed]
- 58.Ray K.K., Kastelein J.J., Boekholdt S.M., Nicholls S.J., Khaw K.T., Ballantyne C.M. The ACC/AHA 2013 guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular disease risk in adults: the good the bad and the uncertain: a comparison with ESC/EAS guidelines for the management of dyslipidaemias 2011. Eur Heart J. 2014;35(15):960–968. doi: 10.1093/eurheartj/ehu107. [DOI] [PubMed] [Google Scholar]


