Abstract
Culicoides sonorensis (Wirth & Jones) is the principal North American vector of bluetongue virus (BTV). BTV infection of livestock is distinctly seasonal (late summer and fall) in temperate regions of the world such as California, which has led to speculation regarding vertical transmission of the virus within the midge vector as a potential mechanism for interseasonal maintenance (“overwintering”) of the virus. To evaluate potential vertical transmission of BTV in its midge vector, we fed adult midges BTV-spiked blood and used a BTV-specific quantitative reverse transcriptase polymerase chain reaction assay to evaluate parent, egg, and progeny stages of laboratory-reared C. sonorensis for the presence of viral nucleic acid. Whereas BTV nucleic acid was weakly detected in egg batches of virus-fed female midges, virus was never detected in subsequent progeny stages (larvae, pupae, and F1 generation adults). Similarly, BTV was not detected in pools of larvae collected from the waste-water lagoon of a BTV-endemic dairy farm in northern California during the seasonal period of virus transmission. Collectively, these results indicate that BTV is not readily transmitted vertically in C. sonorensis, and that persistence of the virus in long-lived parous female midges is a more likely mechanism for overwintering of BTV in temperate regions.
Keywords: Culicoides sonorensis, bluetongue virus, overwintering, vertical transmission
Introduction
Bluetongue virus (BTV) is the causative agent of bluetongue, an economically important arboviral disease of domestic and wild ruminants that is transmitted to its mammalian hosts by certain species of Culicoides biting midges that occur throughout tropical and temperate regions of the world (Verwoerd and Erasmus 2004, Maclachlan and Mayo 2013). Culicoides sonorensis (Wirth & Jones) is the major vector of BTV in much of North America, including California (Gibbs and Greiner 1994). Within temperate zones such as California, BTV infection of ruminant animals is distinctly seasonal, and the vast majority of infections occur during the late summer and fall months (Mayo et al. 2012). The virus largely disappears from ruminants resident in temperate zones from late fall until mid-summer (mid-November until at least late July in much of the Northern Hemisphere). The precise mechanism responsible for this temporal pattern (July–November) of annual BTV infection of animals in temperate zones remains uncertain, including most notably the mechanism responsible for interseasonal maintenance of the virus (so-called “overwintering”; Nevill 1971, Wilson et al. 2008). We have recently shown that BTV likely overwinters in northern California in long-lived parous female midges infected during the prior seasonal period of virus transmission (Mayo et al. 2014). It remains uncertain, however, whether or not transovarial, or vertical, transmission of BTV in vector Culicoides midges can contribute to the process of overwintering of BTV in temperate zones (Nunamaker et al. 1990, White et al. 2005). The current study was undertaken to evaluate potential vertical transmission of BTV in both laboratory-reared and field populations of C. sonorensis midges.
Materials and Methods
Field Investigations
C. sonorensis larvae were collected biweekly from a waste-water lagoon of a dairy farm in northern California that was previously determined to have substantial seasonal BTV transmission (Mayo et al. 2014). Surface mud samples (30 ml) collected from the lagoon edge during August 2012 until August 2013 (∼20 locations per sample date) were pooled and returned to the laboratory under refrigerated conditions (∼2°C for 2 h) where they were warmed to room temperature, thoroughly homogenized, and a 30-ml aliquot processed as previously described (Mayo et al. 2014). Briefly, saturated MgSO4 (Epsom Salts, 100 ml) was added to the sample, stirred, and the live larvae strained from the surface solution 3 min later (Mullens and Lii 1987). Larvae were identified under a dissecting microscope and categorized and counted by instar, according to head capsule size (Mullens and Lii 1987). Larval stages of C. sonorensis from the same collection date were pooled into groups of 50 larvae by instar (L1-2, L3, and L4) for processing.
Laboratory Rearing and Infection of C. sonorensis
A colony of C. sonorensis (VR strain, initially from a dairy farm in San Bernardino County, CA, 1995) was established at UC Davis and reared using methods adapted from Hunt et al. (1994). A strain of BTV serotype 17 previously isolated from a sheep in Modesto County (virus designated as “Modesto 22”), and passaged twice in bovine pulmonary artery endothelial cells, was used for oral infection of female midges at 2-3 d of age, essentially as previously described (Bonneau et al. 2002). Briefly, food (10% sucrose solution) and water were withheld from female midges for 24 h before blood feeding. Midges were infected by feeding on BTV (titer of 105 (tissue culture infectious dose 50) TCID50/ml)-spiked, heparinized cattle blood using a membrane feeder (Supp material [online only]). Midges were allowed to feed for 2 h, after which engorged females were transferred to one of four holding containers in groups of <20 midges. These females were maintained with access to 10% sucrose at 27°C, 40–60% relative humidity, and a photoperiod of 13:11 (L:D) h. A moist filter paper pad was provided daily to the females for oviposition, beginning 2 d after the initial blood or virus feeding. Every two days, deceased females and an aliquot of ∼10% of the total eggs were collected and stored in RNAlater Solution (Ambion, Grand Island, NY) at 4°C for 24 h prior to freezing at −80°C until assayed. The remaining eggs were transferred to larval rearing containers (small pans of nutrient-rich water with polyester batting pads as substrate) specific to their collection day and group. Each egg group was allowed to mature through larval, pupal, and adult stages, and subsets (∼30%) of each stage were collected for virological assay. Surviving females were allowed to feed on uninfected cattle blood (every 4 d) to encourage continued oviposition until the termination of the study (day 16).
Diagnostics
Field-collected larvae were homogenized in lysis binding buffer (AM8500, Ambion/Life Technologies, Grand Island, NY) using stainless steel beads in a tissue homogenizer (Bullet Blender STORM, Next Advance Inc., Averill Park, NY), as previously described (Mayo et al. 2014). All midge life stages (eggs, larvae, pupae, and adults) collected during the laboratory studies were placed in TRI Reagent Solution (Ambion) in a Lysis Matrix I (MP Biomedicals LLC., Santa Ana, CA) system and homogenized by means of a Mini-Beadbeater-16 (Bio Spec Products Inc., Bartlesville, OK) platform. Female midges and their progeny were pooled by life stage and tested in groups of ≤5 individuals from the same testing day and group. Viral RNA was extracted using a MagMAX-96 for Microarrays Total RNA Isolation Kit (Ambion) according to the manufacturer’s recommendations. The presence of BTV nucleic acid in the homogenized samples was determined by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR) amplification and detection of the BTV S10 gene, as previously described (Mayo et al. 2012, 2014). The mean Ct value was calculated for each midge life stage (adult, egg, larva, and pupa) that had Ct values <40. Control samples included in the qRT-PCR assay were extracts of engorged C. sonorensis that fed on cattle blood either spiked or not spiked with BTV, BTV-infected cell culture lysate, and deionized water.
Results
A total of 2,171 field-collected larvae from 15 dates (16 August 2012–22 November 2012, and 23 May 2013–15 August 2013) were processed. No field-collected larval samples were positive for BTV nucleic acid by qRT-PCR; all had Ct values > 40.
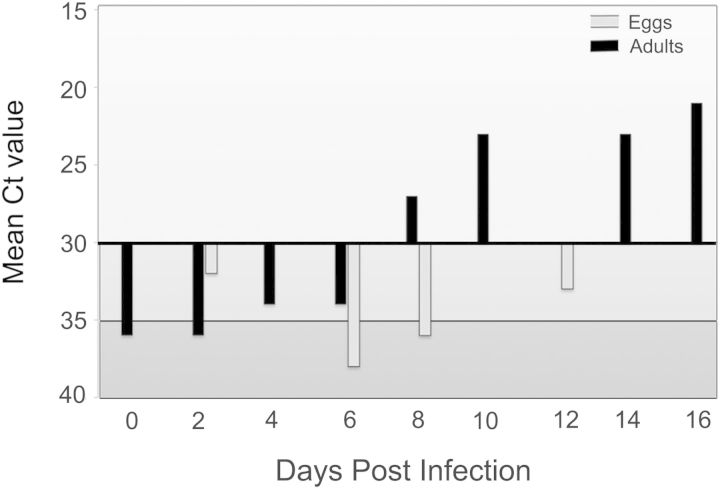
The BTV-spiked cattle blood used for oral infection of adult C. sonorensis had a mean Ct value of 35.5 ± 0.71, as compared with the original cell culture-propagated virus suspension that had a Ct value of 28 ± 4.04. Engorged female midges fed on the BTV-spiked blood also had a Ct value of 36 immediately after blood feeding. Of the 68 females that fed on the BTV-spiked blood, five survived to day 16 postinfection (PI) with a final Ct of 21. These females were likely to be infectious (disseminated infection), as a Ct value < 30 correlates with the presence of infectious virus in this assay (Veronesi et al. 2013, Mayo et al. 2014). Of the females assayed during the incubation period, 89.7% (n = 61) had Ct values <40 and 32.4% of the engorged midges (n = 22) had Ct values < 30, indicating they likely had disseminated BTV infection. After attrition earlier in incubation, this level of infection in surviving females suggests a relatively competent population. The first distinctly BTV-positive flies were > 7 d postfeeding (Fig. 1), consistent with the predicted interval for disseminated infection at a 27°C incubation temperature (Wittmann et al. 2002). Of the 12 egg batches collected over the 16 d of the study, six tested positive for BTV RNA (Fig. 1). Progeny of 11 selected egg batches were used to evaluate potential transtadial and transovarial transmission of BTV; however, BTV was not detected in any of the 115 fourth-instar larvae, 195 pupae, or 135 F1 generation adults (all with Ct values > 40).
Fig. 1.
Detection of BTV RNA by qRT-PCR assay among female C. sonorensis midges and their eggs from 0–16 d after oral infection. Black histograms represent mean cycle threshold (Ct) values of adult midges and gray histograms represent Ct values of eggs oviposted by these midges. The scale on the y-axis is inverted where 15 represents the upper limit and 40 the lower limit for each date. The horizontal solid line indicates the threshold for interpretation of a positive result (light gray; Ct range 15–30), whereas Ct values below this line are interpreted as indeterminant (medium gray; Ct range 30–35) or negative (dark gray; Ct range 36–40).
Discussion
Vertical transmission of BTV to the progeny of C. sonorensis females infected during the period of seasonal virus transmission would offer a compelling mechanism for the interseasonal maintenance of the virus. However, definitive data supporting this hypothesis are lacking (Nunamaker et al. 1990). In contrast to the findings of White et al. (2005) who detected BTV RNA in a substantial proportion of the C. sonorensis larvae collected in Colorado using a nested RT-PCR assay, we did not identify BTV in the progeny of either laboratory-infected midges or C. sonorensis larvae collected during late summer and fall on a seasonally BTV-endemic dairy farm in northern California. Interestingly, some low-level BTV RNA was associated with egg batches of orally infected, laboratory-reared midges, but not in their subsequent progeny. This finding suggests that BTV is disseminated to the accessory glands of infected female midges, and then to the egg surface via the accessory gland fluid during oviposition (Nunamaker et al. 1990, Nelms et al. 2013).
In summary, our combined field and laboratory studies suggest that BTV is not readily transmitted vertically in C. sonorensis, and that persistence of the virus in long-lived parous female midges is a more likely mechanism for overwintering of BTV in temperate regions (Mayo et al. 2014). This conclusion is further supported by findings of our field studies on dairy farms in northern California where BTV infection has been detected only in parous female midges, and never in males or nulliparous females as would be expected if vertical transmission of BTV occurred frequently in the vector (Mayo et al. 2012, 2014). However, additional field studies are warranted, as the efficiency of vertical transmission of virus within the insect vector in other arboviral diseases can be influenced by viral strain, rearing temperature, vector species and strain, and number of gonotrophic cycles completed by infected females (Mullens et al. 2004, Lysyk and Danyk 2007).
Supplementary Data
Supplementary data are available at Medical Entomology online.
Acknowledgments
We gratefully acknowledge producers, staff, and herd managers of the farms from which the samples were obtained. These studies were supported by funds provided by the Agriculture and Food Research Initiative competitive grant 2012-67015-19527 from the U.S. Department of Agriculture–National Institute of Food and Agriculture, and the Center for Food Animal Health at the University of California-Davis, the U.S. Department of Agriculture under the Animal Health Act, 1977, Public Law 95-113.
References Cited
- Bonneau K. R., DeMaula C. D., Mullens B. A., Maclachlan N. J. 2002. Duration of viraemia infectious to Culicoides sonorensis in bluetongue virus – infected cattle and sheep. Vet. Microbiol. 88: 115–125. [DOI] [PubMed] [Google Scholar]
- Gibbs E. P., Greiner. E. W. 1994. The epidemiology of bluetongue. Comp. Immunol. Microbiol. Infect. Dis. 17: 207–220. [DOI] [PubMed] [Google Scholar]
- Hunt G. J. 1994. A procedural manual for the large-scale rearing of the biting midge, Culicoides variipennis (Diptera: Ceratopogonidae), p. 68. USDA Agricultural Research Service ARS-121. [Google Scholar]
- Lysyk T. J., Danyk T. 2007. Effect of temperature on life history parameters of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in relation to geographic origin and vectorial capacity for bluetongue virus. J. Med. Entomol. 44: 741–751. [DOI] [PubMed] [Google Scholar]
- MacLachlan N. J., Mayo C. E. 2013. Potential strategies for control of bluetongue, a globally emerging, Culicoides-transmitted viral disease of ruminant livestock and wildlife. Antiviral Res. 99: 79–90. [DOI] [PubMed] [Google Scholar]
- Mayo C. E., Gardner I. A., Mullens B. A., Barker C. M., Gerry A. C., Guthrie A. J., MacLachlan N. J. 2012. Anthropogenic and meteorological factors influence vector abundance and prevalence of bluetongue virus infection of dairy cattle in California. Vet. Microbiol. 155: 158–164. [DOI] [PubMed] [Google Scholar]
- Mayo C. E., Mullens B. A., Reisen W. K., Osborne C. J., Gibbs E.P.J., Gardner I. A., MacLachlan N. J. 2014. Seasonal and interseasonal dynamics of bluetongue virus infection of dairy cattle and Culicoides sonorensis midges in Northern California - implications for virus overwintering in temperate zones. PLoS ONE 9: e106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullens B. A., Lii K. -S. 1987. Larval population dynamics of Culicoides variipennis (Diptera: Ceratopogonidae) in southern California. J. Med. Entomol. 24: 566–574. [DOI] [PubMed] [Google Scholar]
- Mullens B. A., Gerry A. C., Lysyk T. J., Schmidtmann E. T. 2004. Environmental effects on vector competence and virogenesis of bluetongue virus in Culicoides: interpreting laboratory data in a field context. Vet. Ital. 40: 160–166. [PubMed] [Google Scholar]
- Nelms B. M., Fechter-Leggett E., Carroll B. D., Macedo P., Kluh S., Reisen W. K. 2013. Experimental and natural vertical transmission of West Nile virus by California Culex (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 50: 371–378. [DOI] [PubMed] [Google Scholar]
- Nevill E. M. 1971. Cattle and Culicoides biting midges as possible overwintering hosts of bluetongue virus. Onderstepoort J. Vet. Res. 38: 65–71 [PubMed] [Google Scholar]
- Nunamaker R. A., Sieburth P. J., Dean V. C., Wigington J. G., Nunamaker C. E., Mecham J. O. 1990. Absence of transovarial transmission of bluetongue virus in Culicoides variipennis: immunogold labeling of bluetongue virus antigen in developing oocytes from Culicoides variipennis (Coquillett). Comp. Biochem. Physiol. A Comp Physiol. 96: 19–31. [DOI] [PubMed] [Google Scholar]
- Veronesi E., Antony F., Gubbins S., Golding N., Blackwell A., Mertens P. P., Brownlie J., Darpel K. E., Mellor P. S., Carpenter S. 2013. Measurement of the infection and dissemination of bluetongue virus in Culicoides biting midges using a semi-quantitative rt-PCR assay and isolation of infectious virus. PLoS ONE 8: e70800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verwoerd D.W., Erasmus. B. J. 2004. Bluetongue. In: Coetzer J. A., Tustin R. C. (eds.), Infectious diseases of livestock, 2nd ed, pp. 1201–1220. Oxford University Press, Cape Town, South Africa. [Google Scholar]
- White D. M., Wilson W. C., Blair C. D., Beaty B. J. 2005. Studies on overwintering of bluetongue viruses in insects. J. Gen. Virol. 86: 453–462. [DOI] [PubMed] [Google Scholar]
- Wilson A., Darpel K., Mellor P.S. 2008. Where does bluetongue virus sleep in the winter? PLoS Biol. 6: e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann E. J., Mellor P. S., Baylis M. 2002. Effect of temperature on the transmission of orbiviruses by the biting midge, Culicoides sonorensis. Med. Vet. Entomol. 16: 147–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.