Figure 2.
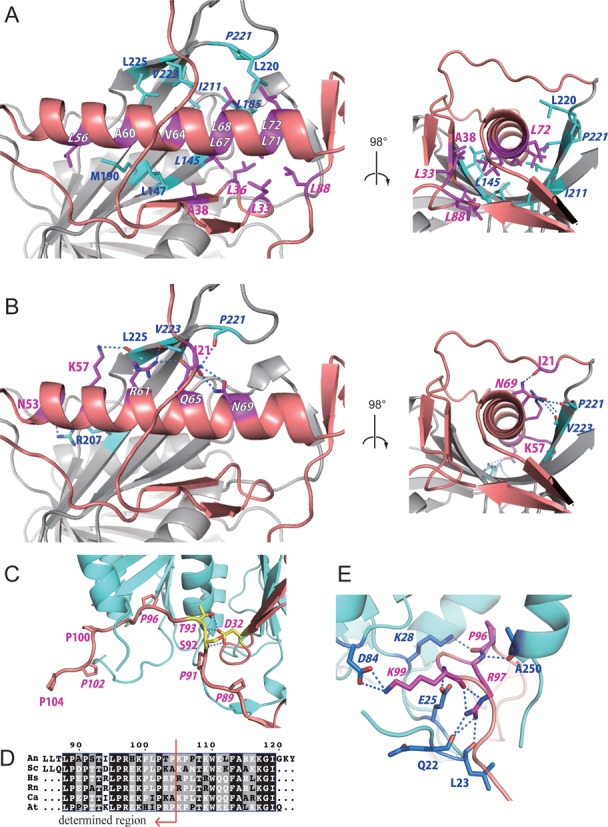
Interactions between Rpf2 and Rrs1. (A and B) Dimerization interface between the long α-helix of Rrs1 and wide β-sheet of Rpf2. Residues of Rrs1 (purple) and Rpf2 (cyan) involved in the interactions are displayed as stick models and labeled. (A) Hydrophobic interactions. (B) Hydrogen-bonding interactions. (C) Close-up view of the C-terminal proline-rich loop of Rrs1. Prolines are labeled and displayed as stick models. Side chains of D32, S92 and T93 from Rrs1 form intra-molecular hydrogen bonds. These three residues are labeled and displayed as yellow stick models. (D) Sequences in the proline-rich loop after CLUSTALW alignment (30). The sequences are as follows: An; Aspergillus nidulans, Sc; Saccharomyces cerevisiae, Hs; Homo sapiens, Rn; Rattus norvegicus, Ca; Candida albicans, At; Arabidopsis thaliana. The most conserved sites are highlighted in black. Red arrow indicates the region for which the structure was determined. (E) Close-up view of hydrogen-bonding interactions at the C-terminal loop of Rrs1. Amino acid residues that form the interaction are displayed as stick models. Conserved residues are denoted in Italics.
