Figure 6.
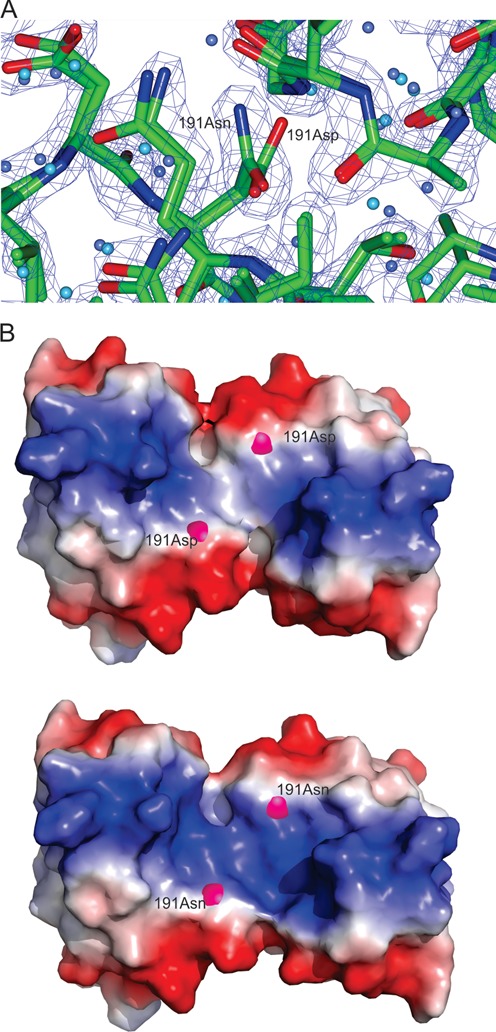
LiaR(DBD)D191N and LiaRDBD homodimer shown as both a cartoon and electrostatic surface representation. (A) Close up comparison of the main and side chain positions of Asp-191 from the LiaR(DBD) and Asn-191 of LiaR(DBD)D191N showed no significant changes in stereochemistry or potentially altered interactions. The (2FOFCWT-PH2FOFCWT) electron density map around Asn191 is contoured at 0.9 absolute value of electrons/Å3. (B). The electrostatic surface (charge surface) of the DNA binding domain of LiaR and LiaRD191N has been calculated. The magenta spheres highlight the position of amino acid 191. Red is negatively charged and blue is positive and there is a color gradient between the two (white being neutral). The DNA binding surface does become slightly more positively charged due to the D191N mutation and therefore more favorable for DNA binding.
