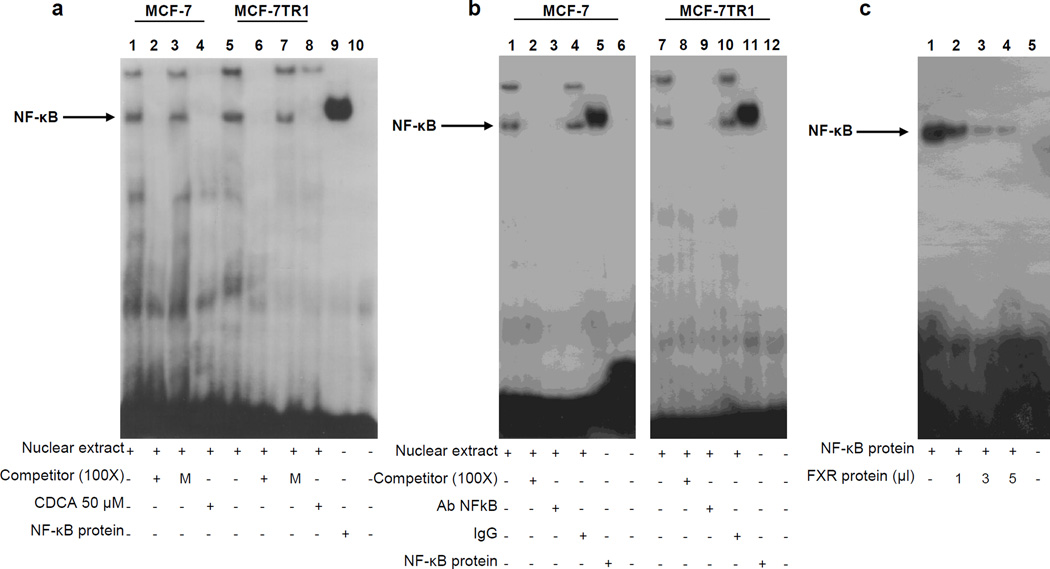
Figure 5.
Electrophoretic mobility shift assay of the NF-κB binding site in the HER2 promoter region. (a) Nuclear extracts from MCF-7 and MCF-7TR1 cells were incubated with a double-stranded NF-κB specific sequence probe labeled with [γ32P]ATP and subjected to electrophoresis in a 6% polyacrylamide gel (lanes 1 and 5). Competition experiments were performed adding as competitor a 100-fold molar excess of unlabeled probe (lanes 2 and 6) or a 100-fold molar excess of unlabeled oligonucleotide containing a mutated NF-κB RE (lanes 3 and 7). Lanes 4 and 8, nuclear extracts from CDCA (50µM) treated MCF-7 and MCF-7TR1 cells respectively incubated with probe. Lane 9, NF-κB protein. Lane 10, probe alone. (b) Nuclear extracts from MCF-7 and MCF-7TR1 cells were incubated with a double-stranded NF-κB specific sequence probe labeled with [γ32P]ATP (lanes 1 and 7) or with a 100-fold molar excess of unlabeled probe (lanes 2 and 8). Nuclear extracts incubated with anti-NF-κB (lanes 3 and 9) or IgG (lanes 4 and 10). Lanes 5 and 11, NF-κB protein. Lanes 6 and 12, probe alone. (c) Lane 1, NF-κB protein. Lanes 2, 3 and 4, NF-κB protein incubated with increasing doses (1, 3 and 5 µl) of transcribed and translated in vitro FXR protein. Lane 5, probe alone.