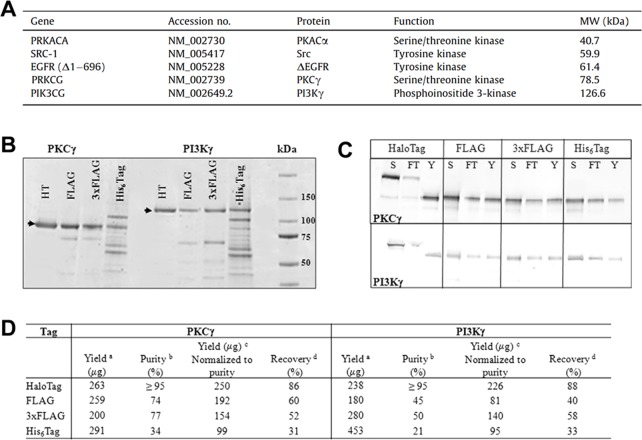
Figure 2.
Functional human kinases isolated and purified from HEK-293 cells using the HaloTag platform. (A) Five human kinases were selected for isolation and purification. (B) To compare the efficiency of HaloTag to that of other protein tagging systems, PKCγ and PI3Kγ were transiently expressed in HEK-293 cells using four protein labeling protocols, including HaloTag, FLAG, 3× FLAG, and His-Tag. Purified proteins were analyzed by SDS-PAGE. Each protein tagging method resulted in purified protein, yet only HaloTag displayed a single band. The arrow denotes the expected molecular weight of the protein. (C) Protein recovery was determined using normalized volumes of soluble lysate (S), unbound fractions (FT), and purified protein (Y) with the addition of a protease using SDS-PAGE and western blot analysis. (D) The HaloTag platform provided the highest purity of protein for both kinases, as compared to that with the other systems. The percent recovery was also shown to be much higher for the HaloTag system. Reprinted with permission from ref (20). Copyright 2011 Elsevier.