Abstract
A practical, chemoselective oxidation of alcohols employing catalytic quantities of DDQ as the oxidant and Mn(OAc)3 as the co-oxidant is described. Electron–rich benzylic alcohols are oxidized efficiently to their corresponding carbonyls, but less electron–rich benzylic alcohols remain unchanged. Allylic alcohols are rapidly oxidized to their corresponding aldehyde or ketone counterparts in high yields. This protocol is operationally simple, employs an inexpensive source of Mn(OAc)3, has short reaction times, and exhibits a significant chemoselectivity favoring allylic alcohols over benzylic alcohols. This procedure also avoids the use of the very large excesses of reagents and sometimes poor reproducibility that characterize previously developed reagents such as MnO2.
Graphical abstract
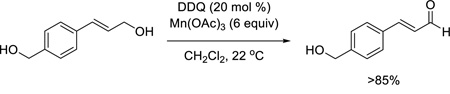
Over the years, a plethora of reagents and conditions have been developed for the mild oxidation of alcohols to carbonyl compounds.1 Some of the many available reagents are chemoselective for specific classes of alcohols. A classic example is MnO2, which has long served as a reagent for the oxidation of benzylic or allylic alcohols.2 Although in very common use, MnO2 requires proper activation to obtain acceptable yields reproducibly, the reactions are often very slow, and they typically employ ten-fold or larger excesses of the reagent.
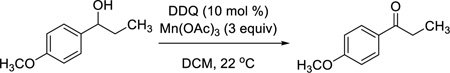
We recently became interested in seeking alternative conditions when we had a need to effect the transformation exemplified in eq 1. Traditional methods
 |
(1) |
(i.e., Swern oxidation,3 Dess-Martin periodinane,4 IBX,5 or MnO2) either provided inadequate yields of the desired product or failed completely. DDQ, 6 however, provided the desired product in 95% yield in less than 10 min. While we were initially encouraged by this result, the high cost of DDQ discourages its use in large-scale operations. We therefore sought a protocol that would allow the catalytic use of DDQ in the presence of a less expensive stoichiometric co-oxidant. Recently, Floreancig reported the use of catalytic amounts of DDQ in the presence of excess MnO2 for other types of oxidative transformations, including cyclizations of ether-containing enol esters to form pyranones, aromatizations, and O-PMB deprotections.7 Mn(OAc)3, which functions as a mild single electron acceptor,8 has also been used for regeneration of DDQ in the removal of PMB protecting groups.9 While this previous work did not include the oxidation of alcohols, we wished to determine whether this or a similar protocol would be amenable to our needs.. As a result of investigating this question, we are now pleased to report a new, modified catalytic oxidation procedure that is simple to perform, provides short reaction times, utilizes a readily prepared co-oxidant, and is not only selective for allylic and benzylic alcohols but which also exhibits selectivity for allylic alcohols in the presence of benzylic alcohols.
Based upon the preceding background, we chose to employ Mn(OAc)3 as the co-oxidant along with catalytic DDQ. While the cost of commercially available Mn(OAc)3 is rather high, we routinely prepare batches of greater than 40 g from inexpensive Mn(OAc)2.10 The reagent is air- and moisture-stable and can be stored in ordinary glassware exposed to air for months at a time.
Our studies began with the aforementioned oxidation of 1-(4-methoxyphenyl)ethanol, an electron-rich benzylic alcohol, with 1.1 mol-equiv of DDQ by itself as a benchmark (Table 1, entry 1). We then examined systematically the effect of Mn(OAc)3, beginning with the use of 3 mol-equiv of this co-oxidant. As expected, decreasing the amount of DDQ resulted in a drastic decrease of product yield (entries 2–6). Even DDQ loadings as high as 50 mol % did not lead to significant product formation. We therefore examined the effect of doubling the amount of Mn(OAc)3 to 6 mol-equiv. Under these conditions, we could lower the amount of DDQ necessary for the reaction to 20 mol % (entry 7). Lower catalyst loadings of DDQ resulted in lower yields. To demonstrate which species is the active oxidant, we conducted the same reaction without DDQ (entry 8). No oxidation product was observed, even after extended reaction times, indicating that DDQ is the active oxidant in the reaction mixture and that the Mn(OAc)3 does indeed serve to regenerate the benzoquinone. It is noteworthy that the loadings of DDQ (20 mol %) and cooxidant (6 mol-equiv) are equal to those reported by Floreancig for quite different transformations.7 Under these conditions, we obtained consistently reproducible results when Mn(OAc)3 was used free from excess acetic acid remaining from the preparation of the reagent.10
Table 1.
Optimization of DDQ oxidation.
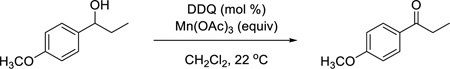 | ||||
|---|---|---|---|---|
| entry | DDQ (mol %) | Mn(OAc)3 (equiv) | time (h) | yielda |
| 1 | 110 | 0 | 0.2 | 95%b |
| 2 | 50 | 3 | 12 | 55% |
| 3 | 20 | 3 | 12 | 45% |
| 4 | 15 | 3 | 12 | 37% |
| 5 | 10 | 3 | 12 | 20% |
| 6 | 5 | 3 | 12 | <10% |
| 7 | 20 | 6 | 3 | 100% (79%)b |
| 8 | 0 | 6 | 12 | 0% |
Footnotes:
Yield based on 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard.
Isolated, purified yield.
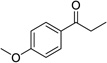
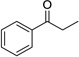
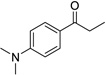
Having optimized this initial example of the catalytic DDQ oxidation, we next tested the scope and limitations of these conditions for benzylic and allylic alcohols. As can be seen in Table 2, a distinct pattern of reactivity emerges for benzylic alcohols. Activated systems, i.e., those bearing electron-donating groups, underwent faster conversions and gave higher overall yields than unactivated systems (compare entries 1 and 2). Very activated systems, such as p-dimethylaminophenylpropanol (entry 3), gave superior results; high yields were obtained in reaction times of typically 6 h or less. A biphenyl substrate (entry 4) underwent smooth oxidation to provide the ketone product in good yield, and 9-hydroxyfluorene (entry 5) gave a very high yield of product after 6 h. The reaction failed in the presence of chlorine and nitro substituents and for the heterocyclic systems that were tested (entries 6–9). Allylic alcohols serve as especially good substrates for this oxidation procedure (entries 10–13).6 We were pleased to observe clean, complete conversion of cinnamyl alcohol to cinnamaldehyde (entry 10). 2-Cyclohexenol underwent clean oxidation in approximately 90% yield (entry 11). An acyclic secondary allylic alcohol proved to be a very good substrate (entry 13).
Table 2.
Substrate Scope of the Mn(OAc)3/Catalytic DDQ Oxidation.
| entry | substrate | product | time (h) | yielda |
|---|---|---|---|---|
| 1 | 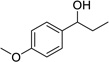 |
 |
3 | 79% |
| 2 |  |
 |
12 | 7% |
| 3b | 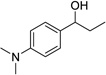 |
 |
1 | 91% |
| 4 | 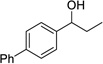 |
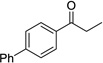 |
4 | 78% |
| 5 | 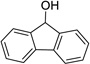 |
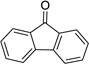 |
6 | 82% |
| 6 | 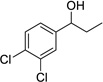 |
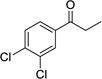 |
12 | 0% |
| 7 | 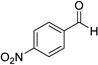 |
12 | 0% | |
| 8 | 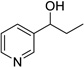 |
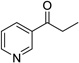 |
12 | 0% |
| 9 | 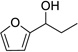 |
12 | 0% | |
| 10 | 2 | >95%c | ||
| 11 | 2 | 90%c | ||
| 12 | 1 | >95%c | ||
| 13 | 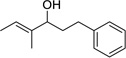 |
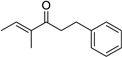 |
2 | 83% |
Footnotes:
Yields refer to isolated, purified products.
2 equivalents of Mn(OAc)3 were used.
Calculated by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
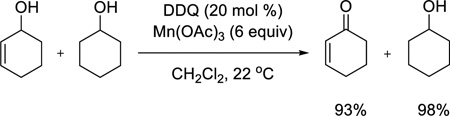
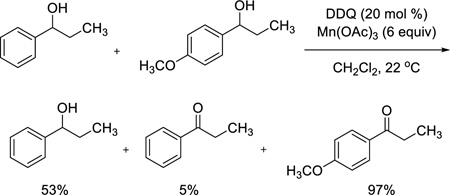
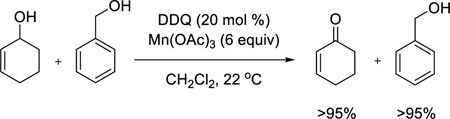
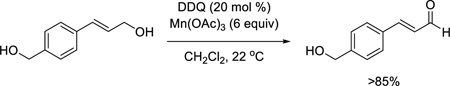
Having observed that allylic alcohols in general and some benzylic alcohols are good substrates for the DDQ/Mn(OAc)3 oxidation, we next conducted competition studies to determine chemoselectivity patterns for different classes of alcohols. Use of a mixture of 2-cyclohexenol and cyclohexanol (eq 2) demonstrates that an aliphatic alcohol remains unchanged while the allylic alcohol is oxidized to the enone in excellent yield. Furthermore, an electron-rich benzylic alcohol is selectively oxidized in the presence of a nonactivated benzylic alcohol (eq 3). Finally, we conducted intermolecular and intramolecular competition studies between benzylic and allylic alcohols (eqs 4 and 5). Due to its sensitivity, the enal product formed in eq 5 was
 |
(2) |
 |
(3) |
 |
(4) |
 |
(5) |
characterized as the corresponding methyl ester (see Supporting Information).11 A very clear chemoselectivity is seen for oxidation of allylic alcohols.
In conclusion, we have developed an alcohol oxidation protocol that utilizes catalytic quantities of DDQ with Mn(OAc)3 as the co-oxidant. The method employs mild conditions and is highly chemoselective. While the process oxidizes certain activated benzylic alcohols selectively, the reaction is most selective for allylic alcohols, which are efficiently converted to the corresponding unsaturated carbonyl compounds. The reaction times are relatively short, ranging from 1 to 6 h for the preferred substrates. Given the highly selective nature of this oxidation, the fast reaction times, and ease with which the reaction can be conducted, we believe that this method will serve as a useful protocol for selective oxidations in multi-step syntheses.
Supplementary Material
Acknowledgment
We would like to thank the Ara Parseghian Medical Research Foundation for providing funding. P.C.V. is grateful for the award of a University of Notre Dame College of Science RESACC Fellowship. A.A.T. is grateful for support from the American Chemical Society Project SEED.
Footnotes
Supporting Information Available. General experimental details, spectral data, and copies of selected 1H NMR and 13C NMR spectra are provided in the Supporting Information.
References
- 1.(a) Modern Oxidation Methods; Bäckvall J-E, editor. Weinheim: Wiley-VCH; 2003. Tojo G, Fernández M. Oxidation of Alcohols to Aldehydes and Ketones: A Guide to Current Common Practice. Springer; 2006.
- 2.Cahiez G, Alami M. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, Burke SD, Denmark SE, Liotta DC, Coates RM, Hart DJ, Danheiser RL, Pearson AJ, Liebskind LS, Reich HJ, editors. New York, NY: Wiley; 1995. pp. 3229–3235. [Google Scholar]
- 3.Tidwell TT. Org. React. 1990;39:297–573. [Google Scholar]
- 4.Tohma H, Kita Y. Adv. Synth. Catal. 2004;346:111–124. [Google Scholar]
- 5.a) Duschek A, Kirsch SF. Angew. Chem., Int. Ed. 2011;50:1524–1552. doi: 10.1002/anie.201000873. [DOI] [PubMed] [Google Scholar]; b) Satam V, Harad A, Rajule R, Pati H. Tetrahedron. 2010;66:7659–7706. [Google Scholar]
- 6.For examples of DDQ being used to oxidize allylic and benzylic alcohols, see: Umezawa I, Nozawa M, Nagumo S, Akita H. Chem. Pharm. Bull. 1995;43:1111–1118. Tempkin O, Abel S, Chen C-P, Underwood R, Prasad K, Chen K-M, Repic O, Blacklock TJ. Tetrahedron. 1997;53:10659–10670. Taber DF, Kanai K. Tetrahedron. 1998;54:11767–11782. Taber DF, Kanai K, Pina R. J. Am. Chem. Soc. 1999;121:7773–7777. Satoh T, Nakamura A, Iriuchijima A, Hayashi Y, Kubota K. Tetrahedron. 2001;57:9689–9696. Belardi J, Curtis L, Clareen S, Shimp H, Leimkuhler C, Simonowicz N, Casillas E. Synth. Commun. 2005;35:1633–1640. Liu S-W, Hsu H-C, Chang C-H, Tsai H-HG, Hou D-R. Eur. J. Org. Chem. 2010:4771–4773. Peng K, Chen F, She X, Yang C, Cui Y, Pan X. Tetrahedron Lett. 2005;46:1217–1220. Sinha AK, Sharma A, Swaroop A, Kumar V. Tetrahedron. 2007;63:1000–1007. Sharma A, Joshi BP, Singh NP, Sinha AK. Tetrahedron. 2006;62:847–851.
- 7.Liu L, Floreancig PE. Org. Lett. 2010;12:4686–8689. doi: 10.1021/ol102078v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snider BB. Chem. Rev. 1996;96:339–363. doi: 10.1021/cr950026m. [DOI] [PubMed] [Google Scholar]
- 9.Sharma GVM, Lavanya B, Mahalingam AK, Krishna PR. Tetrahedron Lett. 2000;41:10323–10326. [Google Scholar]
- 10.Melikyan GG. Synthesis. 1993:833–850. [Google Scholar]
- 11.Riihimäki-Lampén LH, Vainio MJ, Vahermo M, Pohjala LL, Heikura JMS, Valkonen KH, Virtanen VT, Yli-Kauhaluoma JT, Vuorela PM. J. Med. Chem. 2010;53:514–518. doi: 10.1021/jm901309r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


