Table 2.
Substrate Scope of the Mn(OAc)3/Catalytic DDQ Oxidation.
| entry | substrate | product | time (h) | yielda |
|---|---|---|---|---|
| 1 | 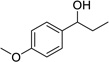 |
 |
3 | 79% |
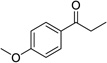
| 2 |  |
 |
12 | 7% |
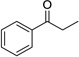
| 3b | 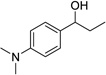 |
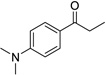 |
1 | 91% |
| 4 | 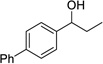 |
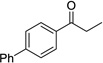 |
4 | 78% |
| 5 | 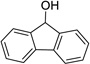 |
 |
6 | 82% |
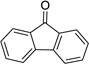
| 6 | 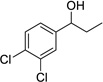 |
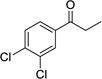 |
12 | 0% |
| 7 | 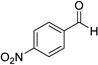 |
12 | 0% | |
| 8 | 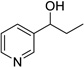 |
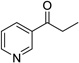 |
12 | 0% |
| 9 | 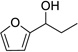 |
12 | 0% | |
| 10 | 2 | >95%c | ||
| 11 | 2 | 90%c | ||
| 12 | 1 | >95%c | ||
| 13 | 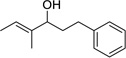 |
 |
2 | 83% |
Footnotes:
Yields refer to isolated, purified products.
2 equivalents of Mn(OAc)3 were used.
Calculated by 1H NMR using 1,3,5-trimethoxybenzene as an internal standard.
