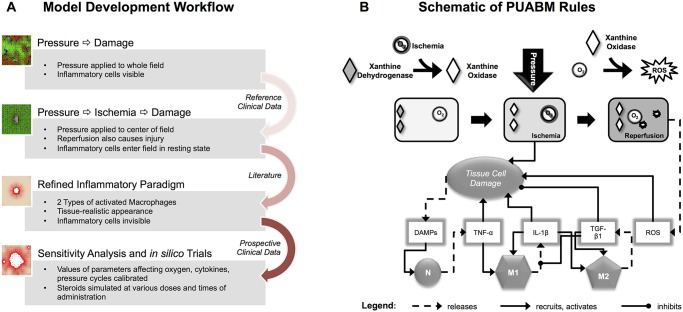
Fig 1. Work flow and schematic of the PUABM.
(A) Work Flow utilized in constructing the PUABM. The ABM was built iteratively, incorporating domain knowledge and data from the literature, then validated with clinical data. Initially, pressure directly injured tissue, thereby inciting inflammation. Ischemia/reperfusion injury was next added as a cause of injury, and the complexity of the inflammatory response was increased. Clinical data were next used to calibrate parameters in the model, and the model was subjected to sensitivity analysis and in silico trials. (B) A schematic illustrates how model components interact to simulate two mechanisms of injury: ischemia/reperfusion injury and damage due to inflammation. A tissue cell (muscle, fat, or skin) is situated over a bony prominence. When pressure is applied, oxygen supply is reduced and the cell becomes ischemic, leading to tissue damage. Simultaneously, the enzyme xanthine dehydrogenase is converted into xanthine oxidase. Thus, reactive oxygen species (ROS) are produced when pressure is released and oxygen flows back to the cell, causing further damage. Tissue damage causes the cell to release DAMPs, which, along with local concentrations of pro-inflammatory cytokines activate both neutrophils (N) and macrophages (M1, M2) when these mediators are present above a given threshold parameter. For example, a sufficiently high local concentration of DAMPs activates neutrophils to secrete TNF-α, which can activate macrophages to a pro- inflammatory M1 phenotype. Tissue damage is ameliorated by anti-inflammatory mediators [TGF-β1].