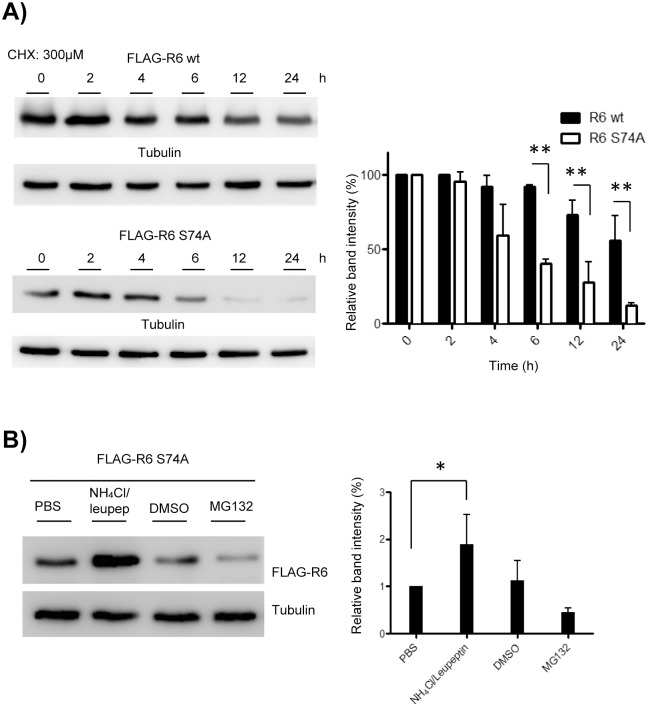
Fig 6. Binding of 14-3-3 proteins to R6 prevents its lysosomal degradation.
A) R6-S74A mutant possesses a shorter half-life than wild type protein. Hek293 cells were transfected with pFLAG-R6 wt or pFLAG-R6-S74A plasmids. 24 hours after transfection, cells were treated with cycloheximide (300 μM) to block protein synthesis. At the indicated times, cell extracts (30 μg) were analyzed by Western blotting using anti-FLAG and anti-tubulin (as loading control). A representative western blot is shown on the left panel. On the right panel, the intensity of the bands related to the levels of tubulin is plotted and normalized respect to the values at time 0 (bars indicate the standard deviation of at least three independent experiments; **p < 0.01). B) R6-S74A protein is degraded by the lysosomal pathway. Hek293 cells were transfected with pFLAG-R6-S74A plasmid. Eighteen hours after transfection, cells were treated with ammonium chloride (20 mM)/ leupeptin (100 μM) or MG132 (5 μM) for six hours. Then, cells were lysed and extracts (30 μg) were analyzed by immunoblotting using anti-FLAG antibody and anti-tubulin as loading control. The intensity of the bands related to the levels of tubulin is plotted (bars indicate standard deviation of at least three independent experiments; *p < 0.05).